Figure 1.
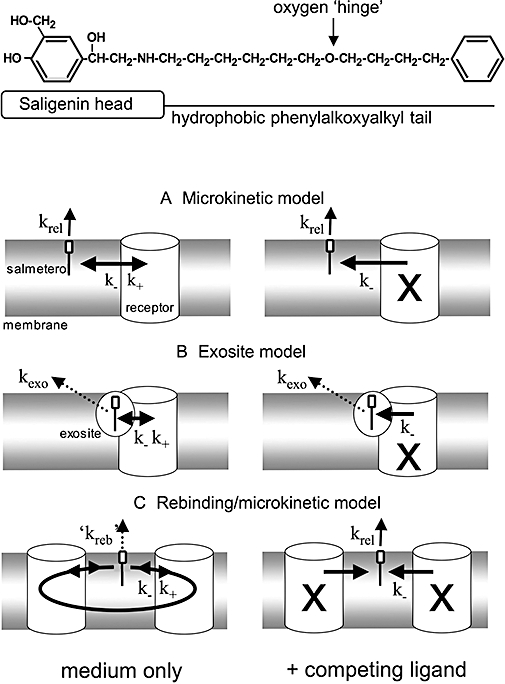
Chemical structure of salmeterol (top) and potential mechanisms for explaining the time-dependencies of its in vitro effects. The saligenin head of salmeterol will bind to the ‘active site’ of the β2-adrenoceptor while the hydrophobic phenylakoxyalkyl tail is important for membrane incorporation and for binding to a potential ‘exosite’. In all models, only membrane- or exosite-associated salmeterol molecules are able to undergo reversible bimolecular interaction with the active site of the receptor (with k+ and k- as association and dissociation rate constants, respectively). Association is prevented when the receptor active sites are occupied by a competing ligand such as a β2-adrenoceptor antagonist (occupied receptors denoted with an X). The following models were tested for their ability to explain the time-related β2-adrenoceptor stimulation by salmeterol under washout conditions either in medium only (left panels) or in medium containing an excess of competing ligand (right panels). (A) Microkinetic model: The membrane acts as a ‘sink’ from where salmeterol is continuously released with the first-order rate constant krel. (B) Exosite model: The side chain of salmeterol binds to an accessory ‘exosite’ located close to or within the β2-adrenoceptor. This allows the saligenin head of salmeterol to continuously sneak in- and out of the active site of the receptor. The membrane still acts as a ‘sink’ but, as the release of salmeterol from the membrane is much faster than its dissociation from the exosite (with first-order rate constant kexo), only the second process was taken into account to calculate the time-wise decline in the amount of salmeterol that can produce receptor stimulation. (C) Rebinding model: This model takes account of the capability of dissociated salmeterol to reassociate to the same or other receptor molecules. The constant shuffling of the involved salmeterol molecules between nearby receptors delays their escape from the membrane. Hence, krel from the microkinetic model is replaced by the much smaller ‘kreb’. Rebinding is prevented in the presence of high concentrations of competing ligand. Under this condition, dissociated salmeterol will escape from the membrane with a rate described by krel.
