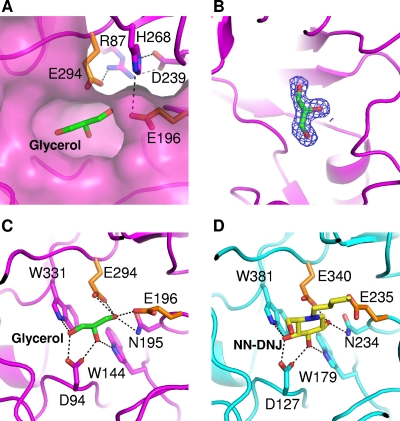
FIG. 3.
The active site and substrate binding of SrfJ. (A) The active site of SrfJ. A pocket-shaped active site is presented in a surface-fill model. Two catalytic residues are presented in a stick model (orange). Residues involved in the hydrogen bond network with the catalytic residues are shown in a stick model in magenta. (B) Experimental electron density map showing the bound glycerol molecule. The 2Fo − Fc electron density (blue mesh) is contoured at 2.0 σ. (C) The mode of glycerol binding to SrfJ. A bound glycerol molecule is shown in a stick model in green. Two catalytic residues are presented as in panel A. Residues involved in glycerol binding are shown in a stick model in magenta. (D) The binding mode of NN-DNJ to human GlcCerase. A bound NN-DNJ molecule is shown in a stick model in yellow. The residues for enzyme catalysis and those involved in NN-DNJ binding are shown in a stick model in orange and cyan, respectively.