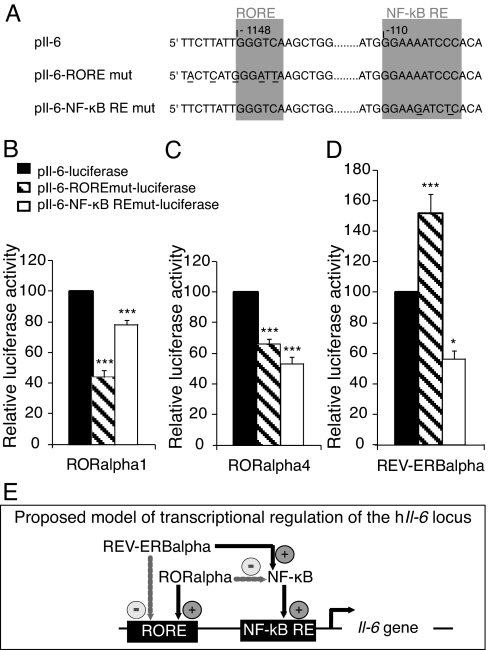
Fig. 5.
Trans-activation of Il-6 by RORα and REV-ERBα. (A) Sequences of native response elements (pIl-6) and response elements point mutated in the RORα (pIl-6-RORE mut) and in the NF-κB (pIl-6- NF-κB RE mut). (B-D) Differential modulation of IL-6 promoter by RORα1, RORα4, and REV-ERBα. Cos-7 cells were transiently co-transfected with reporter plasmids carrying one copy of the native (pIl-6-luciferase) or point-mutated human Il-6 promoters (pIl-6-RORE mut-luciferase or pIl-6-NF-κB RE mut-luciferase) and the expression plasmid vector of the 2 RORα isoforms and of REV-ERBα for 48 h. Transfected cells were then assayed for luciferase activity. Values expressed as percentage of the native reporter pIl-6-Luc are the mean ± SEM of 3 independent experiments with 6 replicates each. Statistical analysis was performed by 1-way ANOVA followed by Scheffé multiple comparison test: *, P < 0.05, **, P < 0.01, ***, P < 0.001, mutated versus native promoter. (E) Proposed model of transcriptional regulation of the human Il-6 promoter. RORα may negatively regulate Il-6 expression through the NF-κB pathway. Alternatively, RORα may trans-activate Il-6 expression by interacting with a RORE in the promoter. For these 2 pathways, RORα competes with REV-ERBα that binds the same response elements with a repressor activity.