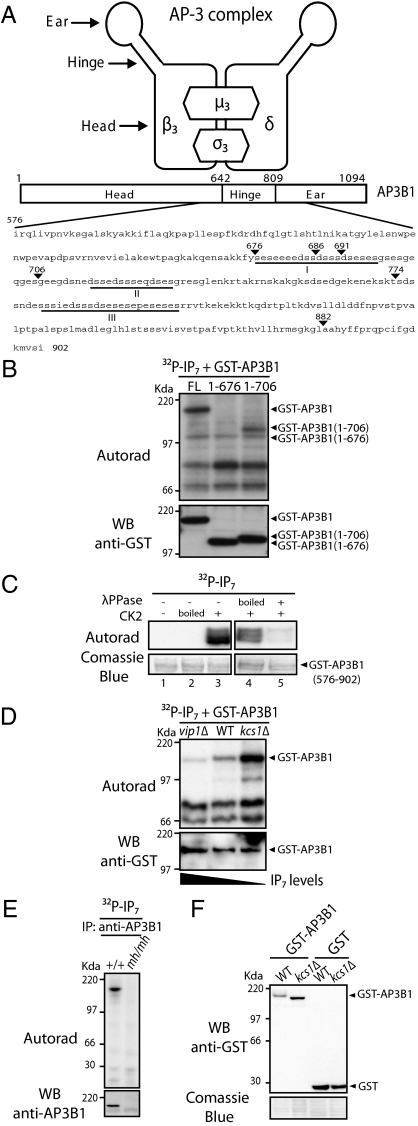
Fig. 1.
AP3B1 is pyrophosphorylated by IP7. (A) Schematic representation of the AP-3 complex and domain structure of AP3B1. The region amino acid 576 to 901 represents the bait used in the yeast two-hybrid screen. The acidic regions containing potential IP7-targeted serines are underlined (regions I–III). (B) In vitro phosphorylation of AP3B1. Protein extracts of kcs1Δ yeast expressing GST-AP3B1 or derivatives were incubated with 5β[32P]IP7 and resolved by NuPAGE; autoradiography was used to determine phosphorylation and immunoblotting with anti-GST antibody confirmed protein equal loading. (C) In vitro pyrophosphorylation of purified AP3B1. GST-AP3B1 (576–902) was expressed and purified from E. coli (BL21). Purified GST-AP3B1 (576–902) immobilized on glutathione beads was preincubated as follows: (i) Without, with inactive (boiled) or with active CK2 and ATP and subsequently treated with 5β[32P]IP7 as in B (lanes 1, 2, and 3, respectively); (ii) with active CK2 and ATP, treated with boiled or active λ-phosphatase, and subsequently phosphorylated with 5β[32P]IP7 as in B (lanes 4 and 5, respectively). (D) In vitro pyrophosphorylation of AP3B1 depends on the endogenous levels of IP7. Protein extracts of vip1Δ, wild-type (WT), and kcs1Δ yeast expressing GST-AP3B1 were incubated with 5β[32P]IP7 and processed as in B. (E) IP7 pyrophosphorylation of endogenous AP3B1. AP3B1 was immunoprecipitated from WT MEF (+/+) or mocha (mh/mh) cell lines, treated with 5β[32P]IP7, and processed as in B. (F) Intracellular pyrophosphorylation of AP3B1 results in a gel mobility shift. Quickly prepared cell extracts from WT and kcs1Δ yeast expressing GST-AP3B1 were boiled in sample buffer and resolved by NuPAGE. Gels are representative of at least three independent experiments.