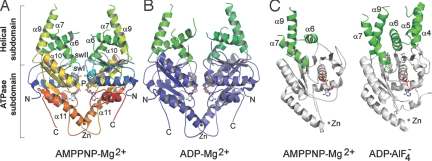
Fig. 1.
Overall structure of Get3. (A) AMPPNP-Mg2+-bound structure of C. therm. Get3. The dimer is in the closed state. N- and C-termini, the P-loop, swI, swII, and the Zn ion and secondary structure elements mentioned in the text are labeled. Coloring of secondary structure elements is done in a ramp from blue (N-terminus) to red (C-terminus). (B) ADP-Mg2+-bound structure of C. therm. Get3. The α-helical (green) and ATPase (blue) subdomains for the 2 chains are colored in similar shades. (C) Overall changes in the α-helical subdomains in different nucleotide states. Monomeric Get3 is shown with AMPPNP-Mg2+ of C. therm. (Left; same for ADP-Mg2+) and with ADP·AlF4− of S. cerevisiae (Right; PDB code 2woj). The α-helical and ATPase subdomains are colored in green and gray, respectively. In the AMPPNP-Mg2+-bound (and ADP-Mg2+-bound) states, the α-helical subdomains are only partially folded.