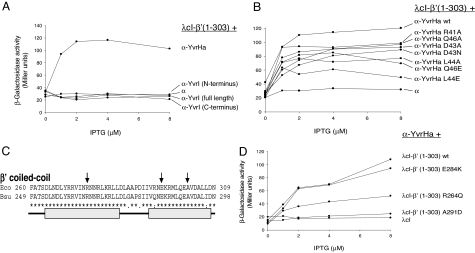
Fig. 4.
YvrHa interacts with the coiled-coil domain in β′-subunit. (A) BACTH analysis of YvrHa and YvrI interactions with β′ (amino acids 1–303). (B) Influence of mutations in the region 2.2-like motif (Fig. 3) of YvrHa on binding with β′ (1–303). (C) Sequence of the E. coli and B. subtilis coiled-coil element in β′ as previously defined (8). Mutations in E. coli β′ (R275Q, E294K, and A302D) that impair β′-binding to σ70-region 2 are indicated with arrows. Approximate dimensions of helices that contribute to the coiled-coil domain are indicated by gray bars. (D) Influence of mutations in B. subtilis β′ (analogous to mutations shown in C) on β′-binding to YvrHa.