Abstract
In response to drought stress the phytohormone ABA (abscisic acid) induces stomatal closure and, therein, activates guard cell anion channels in a calcium-dependent as well as-independent manner. Two key components of the ABA signaling pathway are the protein kinase OST1 (open stomata 1) and the protein phosphatase ABI1 (ABA insensitive 1). The recently identified guard cell anion channel SLAC1 appeared to be the key ion channel in this signaling pathway but remained electrically silent when expressed heterologously. Using split YFP assays, we identified OST1 as an interaction partner of SLAC1 and ABI1. Upon coexpression of SLAC1 with OST1 in Xenopus oocytes, SLAC1-related anion currents appeared similar to those observed in guard cells. Integration of ABI1 into the SLAC1/OST1 complex, however, prevented SLAC1 activation. Our studies demonstrate that SLAC1 represents the slow, deactivating, weak voltage-dependent anion channel of guard cells controlled by phosphorylation/dephosphorylation.
Keywords: ABA signaling, S-type anion channel, OST1/ABI1
Guard cells in the epidermis of plants balance the uptake of CO2 from the atmosphere and the concomitant loss of water from leaves (1–5). When water supply is limited, the drought hormone abscisic acid (ABA) triggers release of anions and K+ from guard cells (6–9). The decrease in guard cell osmotic pressure and volume results in stomatal closure, reducing transpirational loss of water from the leaf.
The initial steps in ABA signal transduction have been shown to activate guard cell anion channels in a calcium-dependent as well as -independent manner (10–13). Elements of these ABA signaling pathways, however, have been identified by genetic screens revealing ABA-insensitive and open-stomata plant mutants with deregulated guard cell volume control (14–17). Among them are protein phosphatases of the PP2C family [ABI1 (ABA insensitive 1) and refs. 2, 14, 18] and a Snf1-related protein kinase 2 [SnRK2.6 named open stomata 1 (OST1)], exhibiting the strongest phenotypes. The type-2C protein phosphatases ABI1 and ABI2 were identified initially on the basis of the ABA-insensitive abi1–1 and abi2–1 dominant mutations (14, 16, 18, 19). Guard-cell activity is impaired in these ABA-insensitive mutants, and, as a consequence, the stomata remain constitutively open even under drought (20). Characterization of loss-of-function alleles indicated that ABI1 and ABI2 are negative regulators of ABA action (21, 22). Using infrared thermography, the Arabidopsis mutants ost1–1 and ost1–2 (SnRK protein kinase family 2) appear “cold” under drought conditions due to their inability to limit their transpiration (17). These recessive ost1 mutations are disrupted in ABA-induced stomatal closure and inhibited in stomatal opening. The Snf1-related kinase 2 (SnRK2) proteins from several plant species have been implicated in ABA signaling pathways (e.g., ref. 15). In Arabidopsis guard cells, OPEN STOMATA 1 (OST1/SRK2E/SnRK2–6) has been described as a critical positive regulator of ABA signal transduction (17, 23). Moreover, OST1 activation in response to ABA is suppressed in the dominant abi1–1 mutant (17), indicating that the protein phosphatase ABI1 (14, 16, 19) negatively regulates ABA signal transduction upstream of OST1. In abi1–1 mutant plants, anion channels fail to respond to ABA (24). From the given information, it seems that the anion channels require phosphorylation for activity (25, 26).
Recently the first guard cell anion channel was identified. An ABA- and CO2/O3-insensitive mutant was shown to lack a gene encoding a putative guard cell anion transporter named SLAC1 (27, 28). In guard cells of these mutant plants, anion currents appeared largely suppressed. When expressed heterologously, SLAC1, however, remained electrically silent (27, 28). Thus to elicit the function of SLAC1 and its role in ABA signal transduction, we searched for partners interacting with this putative anion channel. Using protein–protein interaction assays we identified the protein kinase OST1 and the protein phosphatase ABI1 as regulators of SLAC1 within the ABA transduction pathway (14, 16, 17). When SLAC1 was expressed with OST1 in Xenopus oocytes, SLAC1-related anion currents similar to those observed in guard cells appeared (24, 29). The presence of ABI1, however, prevented SLAC1 activation. Our studies demonstrate that SLAC1 is controlled by OST1/ABI1-dependent phosphorylation/dephosphorylation.
Results
Using gas exchange measurements with intact Arabidopsis leaves, we could show that ost1–2 stomata during day-night transition close much slower than those of WT plants (Fig. S1a). In the light, ost1–2 stomata opened but could not properly adjust their stomatal aperture in response to ongoing water and turgor loss (Movie S1). Lack of OST1-dependent stomatal closure apparently gave rise to deregulated stomatal function and consequently permanent wilting (Fig. S1 b and c, cf. refs. 15, 17, 30 and Movie S1). Previous studies characterized the involvement of ABI1 in regulation of slow guard cell anion channels (e.g., ref. 24). In contrast, such S-type anion channels have not yet been analyzed in the background of ost1 mutants. We therefore examined S-type anion channels in guard cells of ost1 mutant plants and assessed the interaction of SLAC1 with OST1 in vivo. In patch clamp experiments, guard cell protoplasts of Arabidopsis thaliana were loaded with 110 nM cytosolic free Ca2+ and stimulated with ABA. Under these conditions the macroscopic S-type anion currents in open-stomata mutant ost1–2 appeared largely suppressed compared to WT (Fig. 1 A and B).
Fig. 1.
ABA activation of slow anion channels in A. thaliana guard cell protoplasts. (A) Representative macroscopic current responses from WT, ost1–2, and slac1–3 to voltage pulses in the range from + 44 mV to −136 mV are shown. The zero-current level is indicated by the dotted line. (B) Steady-state current densities plotted against the clamped voltages. Data points represent the mean ± SE. The number of protoplasts studied in at least 3 independent experiments were in (B) n = 11 for ost1–2, n = 5 for slac1–3, and n = 12 for WT. The experiments from (A) and (B) were performed in the presence of 25 μM ABA and 110 nM cytosolic free Ca2+.
To test whether these previously identified ABA signaling components are coexpressed with SLAC1 in Arabidopsis guard cells, we performed quantitative real time PCR of SLAC1, OST1 and ABI1 transcripts in guard cells in comparison to the respective mRNA levels in mesophyll cells (Fig. S2; cf. refs. 31, 32). qRT-PCR analysis showed that SLAC1 and OST1 (17) expression appeared to be guard cell specific, while expression of ABI1 was found in both cell types.
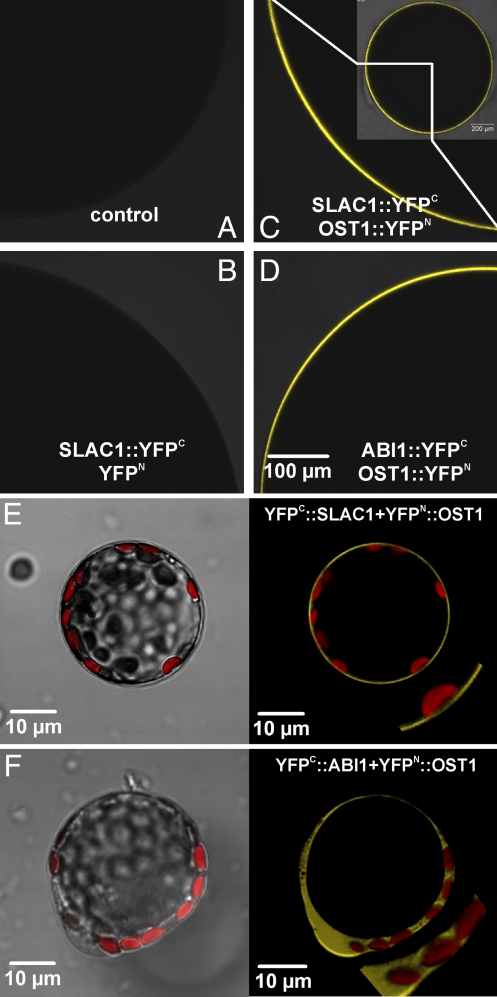
Vahisalu et al. (28) showed that enzymatically isolated guard cell protoplasts from SLAC1 mutants exhibit a largely reduced S-type channel activity (Fig. 1B). We used Xenopus laevis oocytes, a well accepted heterologous expression system for animal, plant, or bacterial channels and transporters, to study whether the gene product of SLAC1 exhibits the S-type anion channel activity in dependence of OST1. The interaction of these putative signaling components was visualized with the bi-molecular fluorescence complementation technique (BiFC, 33). SLAC1 and several protein kinases and phosphatases were fused to a complementary half of split YFP each (for illustration of constructs see Fig. S3). Various cRNA combinations were injected into Xenopus oocytes and analyzed by confocal microscopy (Fig. 2 and Fig. S5). Note, that BiFC experiments in oocytes identify only interaction of partners. Targeting of proteins to the oocyte plasma membrane or cytosol was not distinguished by these fluorescence measurements. When SLAC1, fused to the C-terminal half of the YFP (YFPC), was expressed with the complementary N-terminal half of the YFP (YFPN, Fig. 2B) no specific YFP fluorescence was emitted from oocytes. Upon coinjection of SLAC1::YFPC and YFPN fused to potential interaction partners, fluorescence signals could be detected between SLAC1 and OST1 (Fig. 2C) via complementation of a functional YFP molecule. To exclude the possibility that high expression of 2 proteins in Xenopus oocytes leads to interactions already, we tested close homologues of OST1 (SnRK 2.2/2.3/2.8) together with SLAC1. In contrast to OST1, SnRK 2.2/2.3/2.8 interaction with SLAC1 appeared much weaker (Fig. S5c). In addition, it could be ruled out that the positive BiFC signals might represent nonspecific responses by using the K+ channel GORK together with OST1 as a negative control (Fig. S5b, see Supplemental Text 1). To explore the interaction site between SLAC1 and the kinase we performed BiFC experiments between OST1 and the N and C terminus of SLAC1 (amino acids 1 to 186 and 496 to 556, respectively). YFP complementation could only be monitored between the SLAC1 N terminus and the protein kinase (Fig. S5a). Coinjection of OST1::YFPN with ABI1::YFPC caused YFP emission as well (Fig. 2D), indicating that OST1 directly interacts with ABI1 (34). To investigate the in planta interactions between the ABA signaling components we performed BiFC experiments with Arabidopsis mesophyll protoplasts. After protoplast transformation with YFPC::SLAC1 and YFPN::OST1, YFP-fluorescence complementation was observed at the level of the plasma membrane exclusively (Fig. 2E). As expected for 2 cytosolic proteins, the concerted transformation with YFPN::OST1 and YFPC::ABI1 resulted in YFP fluorescence in the cytosol (Fig. 2F). This in planta assay thus confirmed the data found with the heterologous oocyte expression system.
Fig. 2.
Interactions between proteins involved in the ABA signaling pathway by bimolecular fluorescence complementation (BIFC) in Xenopus oocytes (A–D) as well as Arabidopsis mesophyll protoplasts (E) and (F). Pictures (A–D), taken with a confocal laser scanning microscope, show a quarter of an optical slice of an oocyte (see inset in (C). (A) Noninjected control oocyte. (B) SLAC1::YFPC coexpressed with YFPN. (C) SLAC1::YFPC coexpressed with OST1::YFPN. (D) ABI1::YFPC coexpressed with OST1::YFPN. (E and F) Interaction of SLAC1 and OST1, as well as ABI1 and OST1, monitored in transiently transformed Arabidopsis mesophyll protoplasts by bimolecular fluorescence complementation. (Left) Overlay of transmitted light and chlorophyll fluorescence. (Right) Overlay of YFP and chlorophyll fluorescence. Plasmid combinations were as follows: (E) YFPC::SLAC1 plus YFPN::OST1 and (F) YFPC::ABI1 plus YFPN::OST1.
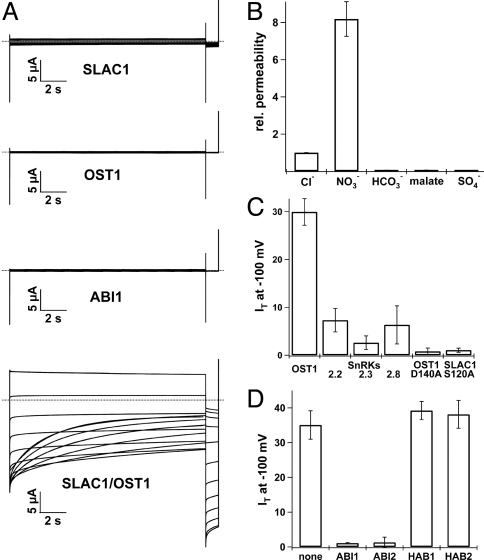
SLAC1 was annotated as a bacteria-like dicarboxylate carrier because of sequence similarities with the yeast MAE1 transporter (27, 28, 35, 36). Complementation and uptake experiments with a malate-transport deficient yeast mutant (27) and SLAC1 expression in oocytes (28), however, did not result in anion transport-competent cells. In two-electrode voltage clamp (TEVC) experiments we thus explored whether SLAC1 cRNA injection in Xenopus oocytes generates a functional anion transporter, when expressed with those ABA signaling components found to interact with the potential anion channel (cf. ref. 37). Oocyte injection with ABI1, OST1 or SLAC1 alone did not result in macroscopic anion currents (Fig. 3A Upper). However, when coexpressed with OST1, currents of up to 50 μA appeared in chloride-based media (Fig. 3A Lower). OST1 activation of SLAC1, however, could be detected in about 25% of the oocyte batches only. In contrast, when using split YFP-fused constructs, SLAC1::YFPC and OST1::YFPN, SLAC1 currents appeared in each single oocyte. Note, that YFP-fusion did not affect the anion channel characteristics (Fig. S4) and that SLAC1 expression alone never led to macroscopic anion currents. Therefore we performed the biophysical characterization of SLAC1-mediated anion currents with the split YFP-fused constructs. Upon application of long lasting voltage pulses to negative membrane potentials, instantaneous SLAC1 currents were recorded, which were followed by a slow deactivation (Fig. 3A Lower, Fig. S6a), anion current characteristics reminiscent to S-type anion currents in intact guard cells (cf. ref. 38). Upon increasing the bath Cl− concentration from 10 to 100 mM the half-maximal activation voltage (V1/2) of SLAC1 channels shifted by 51 mV from −49.6 ± 5.8 mV to −100.4 ± 7.9 mV (Fig. S6c). This result indicates that the voltage dependency of SLAC1 is sensitive to the external chloride concentration. In agreement with an anion-permeable conductance, a reduction of the Cl− concentration in the bath shifted the reversal potential (Vrev) to positive membrane voltages (Fig. S6 b and d). A 10-fold change of the external chloride concentration resulted in a 49.7 ± 1.6 mV shift of Vrev (Fig. S6d). In comparison the anions nitrate and thiocyanate similarly shifted Vrev by −51.4 ± 3.0 mV and −52.9 ± 1.9 mV, respectively, upon a 10-fold change of the external anion concentration (Fig. S6d). Replacement of Na+ by K+ or Li+, however, had no effect on SLAC1 currents and reversal potentials (Fig. S7a). To estimate the permeability of SLAC1 for physiological relevant anions relative to chloride, the halide was replaced by NO3−, SO42−, HCO3− and the dicarboxylate malate and the shift in the respective reversal potentials was determined (Fig. 3B). The derived relative anion permeability sequence of I− (16.92 ± 0.28) > NO3− (8.19 ± 0.44) > Br− (4.02 ± 0.309) > Cl− (1 ± 0.00) ≫ HCO3− (0.05 ± 0.01) > malate− (0.04 ± 0.00) > SO42− (0.04 ± 0.00) characterized SLAC1 as an anion-selective channel with preference to NO3− and Cl− (cf. ref. 39). SLAC1 was isolated in a screen for mutants with defects in stomatal CO2 sensitivity. The impermeability for HCO3− and the lack of HCO3−-induced activity changes of SLAC1 indicate that the anion channel does not sense the CO2 concentration directly. To exclude that acidic bicarbonate or malate buffers could have side-affected the measurements of the SLAC1 conductance, the pH dependence of SLAC1 was analyzed in 100 mM chloride solution. Changing the bath pH from 5 to 6 and 7, SLAC1 activity remained unaffected (Fig. S7b), indicating that protons do not drive chloride transport (cf. 40, 41). In patch clamp studies with plant protoplasts, the anion channel blocker DIDS has previously been shown in plants to inhibit anion currents (42–44). Addition of 100 μM DIDS or SITS to SLAC1-expressing oocytes reduced anion currents at pH 5.6 by 74% and 31%, respectively (Fig. S7c). The effect of DIDS on the Arabidopsis guard cell SLAC1-like anion currents has not been analyzed before. Thus, we analyzed Arabidopsis guard cell protoplasts with patch clamping in the absence and presence of DIDS and could confirm that the stilbene derivative blocks SLAC1-type anion channels in the guard cell background too (Fig. S7c, black bar).
Fig. 3.
Regulation of SLAC1 activity by distinct kinases and phosphatases. (A) Whole-oocyte current recordings in standard bath solution (30 mM Cl−, pH 5.6) upon 15 s voltage pulses ranging from + 40 to −180 mV in 20 mV decrements followed by a 3 s voltage pulse to −120 mV. The holding potential was 0 mV. No prominent current responses of oocytes expressing SLAC1, OST1, or ABI1 alone were recorded (Upper). Only coexpression of SLAC1 together with OST1 (Lower) resulted in macroscopic anion currents slowly deactivating at negative membrane potentials. Representative cells are shown. (B) Relative permeability of SLAC1 for physiological relevant anions (permeability for Cl− was set to 1). Standard bath solution contained 50 mM of the respective anion, pH 5.6 (n = 5). (C) Coexpression studies in oocytes elucidated the specificity of SnRKs toward SLAC1. Instantaneous SLAC1 currents (IT in μA) activated at −100 mV in standard bath solution are shown. Among the tested SnRKs, OST1 coexpression with SLAC1 resulted in the largest anion currents. The coexpression of SLAC1 together with the inactive OST1 mutant D140A prevented SLAC1 currents completely (n ≥ 5). (D) ABI1 and ABI2 coexpression inhibited SLAC1 activation by OST1. Coinjection of HAB1 and HAB2, however, could not prevent SLAC1/OST1 mediated anion currents (n ≥ 3 in μA). Experiments with SLAC1 activated by SnRK/OST1 were performed with oocytes expressing SLAC1::YFPC and SnRK/OST1::YFPN. Error bars in (B–C) represent SD.
Taken together, our findings suggest that the anion channel SLAC1 physically interacts with OST1. Interaction with this protein kinase likely results in SLAC1 phosphorylation and renders the guard cell anion channel active. To test whether phosphorylation is essential for SLAC1 activity, we disrupted the kinase activity of OST1 by introducing an alanine at position 140 instead of the aspartate (see also Supplemental Text 2). When coexpressed with SLAC1::YFPC in the oocyte BiFC system, OST1 D140A::YFPN mutant still complemented YFP fluorescence (Fig. S5b) but failed to activate SLAC1 mediated anion currents (Fig. 3C). Thus SLAC1 activation very likely depends on phosphorylation by OST1. To identify phosphorylation sites of SLAC1, we used peptide arrays of the cytosolic N- and C-terminal part within the SLAC1 protein consisting of 20mer peptides with an overlap of 10 aa (45, 46). After 2 hours of incubation with recombinant OST1 and radiolabeled [γ32P] ATP, we could identify 3 regions of the SLAC1 N terminus, which were phosphorylated by OST1 (position from R41 to L60, from S71 to F90 and from T101 to D130, Table S1). To further confirm this observation, we used a site-directed mutagenesis approach. Thereby all highly predicted serine/threonine phosphorylation sites in the SLAC1 N terminus (predicted by NetPhos2.0; http://www.cbs.dtu.dk/services/NetPhos/ or Scansite motif Scan; http://scansite.mit.edu/motifscan_seq.phtml) were mutated. Testing the respective SLAC1 mutants, we identified residue Ser-120 as an important OST1 target. Replacement of serine 120 by alanine did not affect YFP complementation (Fig. S5b), but SLAC1 could not be activated by OST1 anymore (Fig. 3c). When Ser-120 was substituted by aspartate, to mimic phosphorylation, SLAC1, however, was not active in the absence of OST1. This indicates that Ser-120 represents a critical amino acid residue but its phosphorylation seems to be not sufficient for SLAC1 activation. The specificity of SLAC1 activation by OST1 was tested by using close homologues of OST1. Coexpression of the OST1 homologues kinases SnRK2.2/2.3 and 2.8 activated SLAC1 to a much weaker extend than OST1 (Fig. 3C). These findings are well in line with the results obtained by our BiFC experiments (Fig. S5c).
ABI1 was supposed to act upstream of OST1, repressing OST1 kinase activity upon ABA treatment (17). To test whether potential negative regulators inhibit SLAC1 activation by OST1, we coexpressed SLAC1 and OST1 as well as a set of PP2Cs. When the functional anion channel/kinase complex was coexpressed with the protein phosphatase ABI1 or ABI2 (18, 22), anion currents were abolished (Fig. 3D). In contrast, neither the PP2C HAB1 nor HAB2 (47), could prevent SLAC1 anion currents. Coexpression of OST1 together with ABI1 generated YFP fluorescence (Fig. 2 D and F), whereas OST1 did not show YFP complementation with HAB1 (Fig. S5b). These results of BIFC experiments with OST1::YFPN and ABI1::YFPC or HAB1::YFPC (Fig. 2D and Fig. S5b) are thus supported by the anion current recordings in oocytes (Fig. 3D). Thus ABI1 is capable to inactivate the OST1 pathway by which the guard cell anion channel SLAC1 is activated.
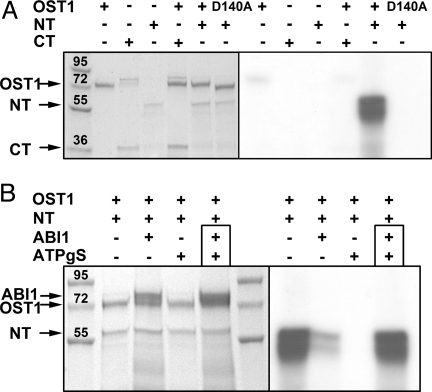
To study the ability of OST1 to phosphorylate SLAC1, we asked whether the recombinant kinase was able to phosphorylate either SLAC1 N or C terminus by in vitro kinase assays. Using radio-labeled [γ32P] ATP, we could show that OST1 phosphorylates the N terminus of SLAC1 exclusively (Fig. 4A). Kinase-inactive OST1 D140A neither showed autophosphorylation nor phosphorylation of SLAC1 NT (Fig. 4A). These results corroborate our oocyte and Arabidopsis protoplast experiments, in which an interaction of OST1 with SLAC1 N terminus but not with SLAC1 C terminus was observed (Fig. S5a). In contrast, no or only a faint phosphorylation of SLAC1 NT by OST1 was observed in the presence of ABI1 (Fig. 4B, compare lane 1 and 2). Consequently, we elucidated, whether ABI1 regulates the activity of OST1 or directly dephosphorylates the N terminus of SLAC1. To prevent phosphorylation of the SLAC1 NT by OST1 we used the ATP analogue ATPγS in surplus (3 mM) relative to ATP (100 μM) (Fig. 4B lane 3). The resulting SLAC1-thio-phosphate-ester is resistant to hydrolysis by phosphatases and would therefore be removed as target of OST1 phosphorylation from the reaction mixture. To elucidate if ABI1 is capable to dephosphorylate the N terminus of SLAC1, we initially phosphorylated SLAC1 NT by OST1 in the presence of [γ-32P] ATP. Subsequently, we added ATPγS and ABI1 to the reaction mixture (Fig. 4B lane 4). Although ATPγS was present to prevent further phosphorylation (cf. Fig. 4B lane 3), ABI1 was not capable to dephosphorylate SLAC1 NT (Fig. 4B lane 4). This indicates that ABI1 acts as a negative regulator of OST1 rather than of the SLAC1 channel.
Fig. 4.
In vitro kinase activity of native recombinant GST1-tagged proteins. (A) Phosphorylation of SLAC1 termini (NT and CT) by OST1 was tested by using radio-labeled [γ32] ATP (Left: Coomassie stained SDS PAGE; Right: radioautogram of the gel; the presence of proteins in the reaction assay was indicated by +). Only SLAC1 NT was phosphorylated by OST1. In contrast to OST1 WT, the OST1 mutant D140A did not phosphorylate SLAC1 NT. Arrowheads indicate the position of recombinant proteins. (B) In vitro kinase assays with native recombinant proteins revealed that OST1 activity was prevented by ABI1 and the ATP analogue ATPγS. When SLAC1 NT was phosphorylated before adding ABI1 together with ATPγS (indicated by the black box, lane 4) we could not detect any dephosphorylation activity of ABI1 within 45 min of incubation at RT. The molecular weight of the protein ladder is indicated in kDa.
Discussion
We could demonstrate by functional expression in Xenopus oocytes that guard cell expressed SLAC1 encodes a weak voltage-dependent, anion-selective plasma membrane channel rather than a malate transporter (27, 28). Furthermore SLAC1 shares neither structural nor functional similarities to AtALMT1, the Arabidopsis orthologue to the wheat aluminum-induced malate channel (48). Thus SLAC1 represents a novel type of inorganic anion channel with unknown tertiary structure, but kinetic properties very similar to the slow anion channel first described with Xanthium strumarium and Vicia faba guard cells (49, 50). Permeability studies with this channel type in V. faba guard cell protoplasts point to a relative malate to chloride permeability of 0.24 (39) which is 6-fold higher than the ratio calculated for SLAC1 expressed in oocytes (Fig. 3B). The putative elevated relative malate permeability in planta likely results from malate transporters expressed in addition to SLAC1 in guard cells such as AtALMT-type carriers or AtABCB14 (48, 51, 52).
In Arabidopsis guard cells, S-type anion channels were reported to be sensitive to the protein kinase inhibitor K252a (26). Furthermore, in guard cells derived from abi1–1 mutant lines that express a deregulated protein phosphatase 2C, S-type anion channels lack ABA activation (24). Here we could show that SLAC1 anion channel interacts with and is activated by the protein kinase OST1. ABI1 functions as a negative regulator of OST1-dependent phosphorylation of SLAC1. Therefore, we suggest that the ABI1 phosphatase targets the OST1 activation rather than the channel dephosphorylation. Recently it was reported that an Arabidopsis triple mutant, with a disruption of 3 strongly ABA-activated protein kinases (SnRK2.2, 2.3 and OST1), is completely insensitive to ABA regarding seed dormancy, germination, seedling growth, and plant transpiration (53, 54). Although the close homologues SnRK2.2 and 2.3 (55) of OST1 were shown to activate SLAC1 as well (to some lesser extent, Fig. 3C), the distinct spatiotemporal expression of these kinases (especially SnRK2.2 and 2.3) likely contributes to their different physiological function.
Several ABA binding proteins (protein families) are currently discussed as putative ABA receptors (56–61). Our previous in planta guard cell studies have shown that cytosolic application of ABA is activating SLAC1-like anion channels instantaneously, whereas external stimulation delays this process (11). This observation points to an ABA importer and internal ABA receptor. Using independent approaches, recently 2 labs identified a new family of ABA-binding proteins (57, 59). These proteins interact with ABI1 and could represent the predicted cytoplasmic ABA receptor (11). In line with our findings, it was shown that the ABA-receptor/ABI1 complex interacts and activates SnRK2 kinases upon ABA-treatment. This fills the gap between ABA perception and SLAC1-mediated membrane depolarization. Based on these findings future reconstitution experiments to decipher fast (constitutive) and slow (transcriptionally-induced) ABA signaling pathways may now appear increasingly feasible (1).
Methods
Real-Time PCR.
Quantification of actin2/8 and SLAC1/OST1/ABI1-transcripts was performed by real-time PCR as described elsewhere (62). Transcripts were each normalized to 10,000 molecules of actin 2/8. Primers are listed in SI Methods.
Oocyte Recordings.
The cDNA of SLAC1, OST1, SnRK2.2, 2.3, 2.8, ABI1, ABI2, HAB1, and HAB2 were cloned into oocyte (BIFC-) expression vectors by an advanced uracil-excision-based cloning technique described by Nour-Eldin et al. (63). Oocyte preparation and cRNA generation and injection have been described elsewhere (64). In TEVC experiments after 2 or 3 days of expression oocytes were perfused with Kulori-based solutions. For detailed information regarding solutions, pulse protocols and data analysis see SI Methods.
BIFC Experiments.
The cDNA of SLAC1, OST1, and ABI1 were cloned into plant binary vectors (based on pCAMBIA vectors) as described by Nour-Eldin et al. (2006) (63). Transient protoplast expression was performed using the polyethylene glycol transformation method modified after (65). Sixteen to 24 h after transformation, protoplast images were taken with a confocal laser scanning microscope (LSM 5 Pascal Carl Zeiss Jena GmbH). For further information see SI Methods.
Patch Clamp Experiments on Guard Cell Protoplasts.
Arabidopsis thaliana ecotype Columbia (Col-0), ost1–2 and slac1–3 mutants were grown on soil in a growth chamber at a 8/16 h day/night regime and 22/16 °C day/night temperature. Enriched protoplasts were stored on ice until aliquots were used for whole-cell patch clamp recordings of S-type anion currents which were performed essentially as described by (66, 67). For details of protoplast generation and patch clamp conditions see SI Methods.
Protein purification and in Vitro Kinase Assays.
OST1, ABI1, SLAC1 NT and CT were subcloned into the recombinant expression vector pGEX 6P1 (GE Healthcare) and transformed into Escherichia coli (DE3) pLysS strain (Novagen). GST-tagged recombinant proteins were purified as described by Belin et al. (68). In vitro kinase buffer was composed of 20 mM Hepes, pH 7.5, 0.5% (vol/vol) Triton X-100, 2 mM MnCl2, protease inhibitor mixture (Roche), 10 mM NaF, 5 mM β-glycerophosphate, and 5 mCi [ϒ-32P] ATP (3,000 Ci/mmol) (cf. ref. 68). Reactions were carried out for 15 min at RT and then stopped by adding 6 × SDS loading buffer and heating to 90 °C for 5 min. Proteins were separated by SDS-Gel electrophoresis using a 8 to 16% gradient acrylamide gel (PreciseTM protein gel, Thermo Scientific) and detected by coomassie blue stain and autoradiography.
CelluSpots peptide arrays were ordered from Intavis (Bioanalytical Instruments); 20 aa long peptides with 10 aa overlap at each end of SLAC1 N and C terminus were coupled to microscope slides by acetylation. To prevent nonspecific binding, the arrays were blocked by immersing the slides in 1 mg/ml BSA solution for 2 h at RT. The phosphorylation reaction was carried out for 2 h at RT by the use of 2 μg kinase, 5 mCi [ϒ-32P] ATP (3,000 Ci/mmol) and in vitro kinase buffer described above. Subsequently slides were washed and phosphorylation was detected by autoradiography.
Supplementary Material
Acknowledgments.
We thank Gregory Harms for critical reading and comments on the manuscript. We gratefully acknowledge H. H. Nour-Eldin and B. A. Halkier for providing us oocyte and plant BIFC vectors. This work was supported by grants from the Deutsche Forschungsgemeinschaft within the research group FOR 964 (to R.H. and T.R.) and GK1342 (to R.H.) and a King Saud University grant (to R.H. and K.A.S.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912021106/DCSupplemental.
References
- 1.Assmann SM, Shimazaki K. The multisensory guard cell. Stomatal responses to blue light and abscisic acid. Plant Physiol. 1999;119:809–816. doi: 10.1104/pp.119.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 3.Raschke K. Action of abscisic acid on guard cells. In: Zeiger GDF E, Cowan IR, editors. Stomatal Funktion. Stanford, CA: Stanford Univ Press; 1987. pp. 253–279. [Google Scholar]
- 4.Roelfsema MR, Hedrich R. In the light of stomatal opening: New insights into “the Watergate”. New Phytol. 2005;167:665–691. doi: 10.1111/j.1469-8137.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- 6.Keller BU, Hedrich R, Raschke K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature. 1989;341:450–453. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebaudy A, Very AA, Sentenac H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 8.MacRobbie EA. Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond B. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard Cell Signal Transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 10.Hetherington AM, Brownlee C. The generation of Ca(2+) signals in plants. Annu Rev Plant Biol. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- 11.Levchenko V, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R. Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc Natl Acad Sci USA. 2005;102:4203–4208. doi: 10.1073/pnas.0500146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marten H, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R. Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum. Plant Physiol. 2007;143:28–37. doi: 10.1104/pp.106.092643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- 14.Leung J, et al. Arabidopsis ABA response gene ABI1: Features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wang XQ, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- 16.Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 17.Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. [Google Scholar]
- 20.Roelfsema MRG, Prins HBA. Effect of abscisic acid on stomatal opening in isolated epidermal strips of abi mutants of Arabidopsis thaliana. Physiol Plant. 1995;95:373–378. [Google Scholar]
- 21.Gosti F, et al. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell. 1999;11:1897–1910. doi: 10.1105/tpc.11.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 2001;25:295–303. doi: 10.1046/j.1365-313x.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 23.Assmann SM. OPEN STOMATA1 opens the door to ABA signaling in Arabidopsis guard cells. Trends Plant Sci. 2003;8:151–153. doi: 10.1016/S1360-1385(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 24.Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen GJ, Kuchitsu K, ChuSP, Murata Y, Schroeder JI. Arabidopsis abi1–1 and abi2–1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt C, Schelle I, Liao YJ, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negi J, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 28.Vahisalu T, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raschke K. Alternation of the slow with the quick anion conductance in whole guard cells effected by external malate. Planta. 2003;217:651–657. doi: 10.1007/s00425-003-1034-3. [DOI] [PubMed] [Google Scholar]
- 30.Merlot S, et al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002;30:601–609. doi: 10.1046/j.1365-313x.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 31.Leonhardt N, et al. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell. 2004;16:596–615. doi: 10.1105/tpc.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9(4):789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida R, et al. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]
- 35.Grobler J, Bauer F, Subden RE, Van Vuuren HJ. The mae1 gene of Schizosaccharomyces pombe encodes a permease for malate and other C4 dicarboxylic acids. Yeast. 1995;11:1485–1491. doi: 10.1002/yea.320111503. [DOI] [PubMed] [Google Scholar]
- 36.Saji S, et al. Disruption of a gene encoding C4-dicarboxylate transporter-like protein increases ozone sensitivity through deregulation of the stomatal response in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:2–10. doi: 10.1093/pcp/pcm174. [DOI] [PubMed] [Google Scholar]
- 37.Emmerlich V, et al. The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA. 2003;100:11122–11126. doi: 10.1073/pnas.1832002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marten H, Hedrich R, Roelfsema MR. Blue light inhibits guard cell plasma membrane anion channels in a phototropin-dependent manner. Plant J. 2007;50:29–39. doi: 10.1111/j.1365-313X.2006.03026.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt C, Schroeder JI. Anion selectivity of slow anion channels in the plasma membrane of guard cells (large nitrate permeability) Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Angeli A, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 41.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 42.Frachisse JM, Colcombet J, Guern J, Barbier-Brygoo H. Characterization of a nitrate-permeable channel able to mediate sustained anion efflux in hypocotyl cells from Arabidopsis thaliana. Plant J. 2000;21:361–371. doi: 10.1046/j.1365-313x.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- 43.Marten I, Busch H, Raschke K, Hedrich R. Modulation and block of the plasma membrane anion channel of guard cells by stilbene derivatives. Eur Biophys J. 1993;21:403–408. [Google Scholar]
- 44.Schroeder JI, Schmidt C, Sheaffer J. Identification of high-affinity slow anion channel blockers and evidence for stomatal regulation by slow anion channels in guard cells. Plant Cell. 1993;5:1831–1841. doi: 10.1105/tpc.5.12.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohmer FD, Uecker A. A substrate peptide for the FLT3 receptor tyrosine kinase. Br J Haematol. 2009;144:127–130. doi: 10.1111/j.1365-2141.2008.07408.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Li SS. CelluSpots: A reproducible means of making peptide arrays for the determination of SH2 domain binding specificity. Methods Mol Biol. 2009;570:197–202. doi: 10.1007/978-1-60327-394-7_8. [DOI] [PubMed] [Google Scholar]
- 47.Saez A, et al. Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J. 2004;37:354–369. doi: 10.1046/j.1365-313x.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 48.Pineros MA, Cancado GM, Kochian LV. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus Oocytes: Functional and structural implications. Plant Physiol. 2008;147:2131–2146. doi: 10.1104/pp.108.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linder B, Raschke K. A slow anion channel in guard cells, activating at large hyperpolarization, may be principal for stomatal closing. FEBS Lett. 1992;313:27–30. doi: 10.1016/0014-5793(92)81176-m. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder JI, Keller BU. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovermann P, et al. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007;52:1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 52.Lee M, et al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol. 2008;10:1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- 53.Fujii H, Zhu JK. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA. 2009;106:8380–8385. doi: 10.1073/pnas.0903144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakashima K, et al. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009;50:1345–1363. doi: 10.1093/pcp/pcp083. [DOI] [PubMed] [Google Scholar]
- 55.Fujii H, Verslues PE, Zhu JK. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell. 2007;19:485–494. doi: 10.1105/tpc.106.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, et al. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 57.Ma Y, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 58.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 59.Park SY, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Razem FA, El-Kereamy A, Abrams SR, Hill RD. The RNA-binding protein FCA is an abscisic acid receptor. Nature. 2006;439:290–294. doi: 10.1038/nature04373. [DOI] [PubMed] [Google Scholar]
- 61.Shen YY, et al. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–826. doi: 10.1038/nature05176. [DOI] [PubMed] [Google Scholar]
- 62.Ivashikina N, et al. Isolation of AtSUC2 promoter-GFP-marked companion cells for patch-clamp studies and expression profiling. Plant J. 2003;36:931–945. doi: 10.1046/j.1365-313x.2003.01931.x. [DOI] [PubMed] [Google Scholar]
- 63.Nour-Eldin HH, Hansen BG, Norholm MH, Jensen JK, Halkier BA. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 2006;34:e122. doi: 10.1093/nar/gkl635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker D, et al. Changes in voltage activation, Cs+ sensitivity, and ion permeability in H5 mutants of the plant K+ channel KAT1. Proc Natl Acad Sci USA. 1996;93:8123–8128. doi: 10.1073/pnas.93.15.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abel S, Theologis A. Transient transformation of Arabidopsis leaf protoplasts: A versatile experimental system to study gene expression. Plant J. 1994;5:421–427. doi: 10.1111/j.1365-313x.1994.00421.x. [DOI] [PubMed] [Google Scholar]
- 66.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 67.Ivashikina N, Deeken R, Fischer S, Ache P, Hedrich R. AKT2/3 subunits render guard cell K+ channels Ca2+ sensitive. J Gen Physiol. 2005;125:483–492. doi: 10.1085/jgp.200409211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belin C, et al. Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol. 2006;141:1316–1327. doi: 10.1104/pp.106.079327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.