Abstract
Objective
To determine whether peak expiratory flow (PEF), when expressed by a validated method using standardized residual (SR) percentile, is associated with subsequent disability and death in older persons.
Design
Prospective cohort study.
Setting
New Haven, Connecticut.
Participants
754 initially nondisabled, community-living persons aged 70 years or older.
Measurements
PEF was assessed at baseline along with chronic conditions and smoking history. The onset of persistent disability in activities of daily living (ADL), continuous mobility disability, and death were ascertained during monthly interviews over a five-year period.
Results
The mean age was 78.4 years; 63.7% had a smoking history and 17.4% reported chronic lung disease. The incidence rates per 100 person-months (95% confidence intervals) were 1.00 (0.90, 1.12) for ADL disability, 0.80 (0.70, 0.93) for mobility disability, and 0.44 (0.38, 0.51) for death. At a PEF < 10th SR-percentile, identifying nearly a quarter of the cohort, hazard ratios (HR) adjusted for multiple confounders, including age, smoking, and chronic lung disease, demonstrated an increased risk of ADL disability (HR [95% confidence interval]: 1.79 [1.23, 2.62]), mobility disability (1.89 [1.15, 3.10]), and death (2.31 [1.29, 4.12]).
Conclusion
In our elderly cohort, we found that a diminished PEF, when expressed as an SR-percentile, is independently associated with subsequent disability and death. These results support the use of PEF as a potentially valuable risk assessment tool among community-living older persons.
Keywords: Peak expiratory flow, standardized residuals, disability, death
INTRODUCTION
Peak expiratory flow (PEF), defined as the maximum flow achieved during expiration delivered with maximal force starting from maximal lung inflation,1 has several attributes that warrant its consideration as a risk assessment tool in older persons. First, it is a simple, inexpensive, and readily available measure of pulmonary function.1 Second, in older persons, PEF is cross-sectionally associated with health status and physical and cognitive function.2–5 Third, a decreased PEF in older persons is longitudinally associated with cognitive decline, institutionalization, and death.6–8 Fourth, in many cases, these associations persist even after accounting for smoking status.2,4,5,8 As discussed below, however, these findings lack generalizability because PEF has not been reported in a manner consistent with published guidelines.1,9–11
In a normal reference population, PEF is influenced by the anthropometric variables of height, age, and gender, and, when appropriate, ethnicity.1,9–11 Weight is not a predictor variable, as obesity does not lead to a larger lung, although it may reduce pulmonary function.12,13 Thus, when reporting PEF, a comparison needs to be made between what is measured and a reference value, namely the predicted mean of a population of normal subjects having the same anthropometric variables.1,9–11 In older persons, such a comparison is expressed effectively as a standardized residual (SR), calculated as [(measured − predicted) / (standard deviation of the residuals)].1,9,14 In this equation, the numerator is referred to as the residual, while the denominator is a constant that quantifies the scatter of measured values about the predicted mean (i.e., the spread of the reference data); a percentile based on the SR is then computed.9,14
Importantly, prior studies in older persons have reported PEF either simply as a measured value, without comparison to a predicted mean;3,4,6,7 or as a residual that is neither standardized nor based on a correctly derived predicted mean, i.e., weight was used as a predictor variable.2,5,8 Furthermore, despite the preeminence of functional outcomes in older persons, prior studies of PEF have not evaluated the association of PEF and subsequent disability.
In the present study, we set out to determine whether PEF, expressed as an SR-percentile, is associated with subsequent disability and death in a cohort of initially nondisabled, community-living older persons. 14 Such findings would support the use of PEF as a risk assessment tool for outcomes that are relevant to independent living and longevity.
METHODS
Study Population
Participants were drawn from an ongoing longitudinal study of 754 initially nondisabled, community-living persons, aged ≥ 70 years. The assembly of the cohort, which took place between March 1998 and October 1999, has been described elsewhere.15 Potential participants were identified from a computerized list of 3,157 age-eligible members of a large health plan in greater New Haven, Connecticut. Eligible participants were community-living, English-speaking, and nondisabled in four key activities of daily living (ADL) – bathing, walking, dressing, and transferring. Persons scoring > 10 seconds on the rapid gait test were oversampled to ensure a sufficient number of participants at increased risk for ADL disability, as described previously.15 Slow gait speed has repeatedly been shown to be the single best predictor of ADL disability.16–18 Health plan members were excluded on the basis of three criteria: diagnosis of a terminal illness with a life expectancy less than twelve months, plans to move out of the New Haven area during the next 12 months, and significant cognitive impairment with no available proxy.15
Eligibility was determined during a screening telephone interview and was confirmed during an in-home assessment. Only 4.6% of the 2,753 health plan members who were alive and could be contacted refused to complete the screening telephone interview, and only 3.6% of otherwise eligible persons were excluded because of significant cognitive impairment and no available proxy. Of the 1002 eligible members, 754 (75.2%) agreed to participate in the study. Persons who refused to participate did not differ significantly from those enrolled in terms of age or sex. The study protocol was approved by the Yale Human Investigation Committee.
Data Collection
At baseline, participants underwent a comprehensive home-based assessment by trained nurse researchers who used standard instruments. Data were collected in several categories, including demographic characteristics and smoking status; nine self-reported, physician-diagnosed chronic conditions, including chronic lung disease; self-reported health; and cognitive status. Chronic lung disease was present if the participant answered either “Yes” to – (Part A) “Has a doctor ever told you that you have chronic lung disease such as chronic bronchitis, chronic obstructive pulmonary disease (COPD), asthma, or emphysema?” or if the participant answered “suspected/possible” to Part A and "Yes" to – (Part B) "Does your lung disease limit your usual activities such as household chores?" Cognitive status was evaluated by the Mini-Mental State Examination,19 with an abnormal score being < 24.
PEF
At baseline, a Mini-Wright meter (Clement Clarke International; Essex, England) measured PEF using a standardized protocol, as previously described.14 Seven hundred and fifty participants (99.5%) completed three PEF readings, with an additional 3 (0.4%) achieving at least one reading, leaving 753 (99.9%) in our study population. The PEF test was largely performed with good-to-excellent understanding (n=702 or 93%); the exclusion of participants with less than good-to-excellent understanding (n=51 or 7%) had no appreciable effect on the results. The highest PEF value (liters/minute) was used in the current analyses, regardless of level of understanding in the performance of the PEF test. Variability in effort was minimal with the intraclass correlation coefficient for the three PEF readings being 0.92 (95% confidence interval: 0.91, 0.93).
As described in more detail elsewhere,14 we expressed the PEF measurements as a standardized residual (SR), calculated as [(measured−predicted) / (standard deviation of the residuals)], and thereafter converted to a SR-percentile. Based on guidelines set forth by the American Thoracic Society and the European Respiratory Society, the predicted mean, including its range of normal, should be determined in a reference population of asymptomatic never-smokers having similar anthropometrics, namely height, age, and gender.1,9–11 Ethnicity was not considered when defining normality since the vast majority (90.4%) of our cohort was non-Hispanic white. Hence, our reference population for the predicted mean was defined as a subgroup of never-smokers who were able to complete three PEF readings and who had no history of lung disease, myocardial infarction, congestive heart failure, stroke or cancer (except for non-melanoma type skin cancers). Separate regression models were fit for the male and female reference groups. The resulting predictive equations were then used to calculate the predicted PEF for the other participants in the cohort.1,9,14
We next classified PEF in categories or “stages” based on SR-percentiles and using cut-points that are consistent with current clinical practice. For example, the National Asthma Education and Prevention Program utilizes PEF cut-points at 80 and 50 percent of one’s personal best to define asthma severity.20 Similarly, the Global Initiative for Obstructive Lung Disease (GOLD) has established a staging scheme for COPD based on the forced expiratory volume in 1 second (FEV1), with cut-points set at 80, 50, and 30 percent predicted.21 Although these thresholds have not been rigorously validated, they nevertheless can be used to identify the curvilinear relationship that is known to exist between spirometric measures of pulmonary function and respiratory symptoms or physical activity.22,23 In the current study, because of the large number of participants with PEF values below the 30th SR-percentile, we added a lower stage threshold. Hence, our staging cut-points were 80, 50, 30, and 10, expressed as SR-percentile.
Outcomes
Our two disability outcomes—persistent ADL disability and continuous mobility disability—were ascertained during monthly telephone interviews that were completed by trained research assistants over the course of five years. Details regarding our assessment of disability, including formal tests of reliability and accuracy, have been described elsewhere.24,25 ADL disability was operationalized as the need for personal assistance or being unable to perform one or more of the four key ADLs, and was considered persistent if present for at least two consecutive months.24 Mobility disability was defined as the need for personal assistance or being unable to walk ¼ mile or climb a flight of stairs, and was considered continuous if present for at least six consecutive months.25 Deaths were ascertained by review of local obituaries and/or from an informant. Death certificates were obtained for 174 (99%) of the 176 decedents and the immediate cause of death was coded by a professional nosologist using the International Classification of Diseases (ICD-10).
Thirty (4.0%) participants dropped out of the study after a median follow-up of 22 months. Data were otherwise available for 99% of the 38,831 monthly telephone interviews.
Statistical Analysis
Incidence rates with 95% confidence intervals for persistent ADL disability, continuous mobility disability, and death were expressed in person-months. The time to onset of each outcome was evaluated using the Kaplan-Meier method with strata defined by baseline PEF stage. The log rank test was used for statistical comparisons. Participants who were lost to follow-up or who did not achieve the respective outcome over the five-year study period were censored, while those who had mobility disability at baseline were excluded for the mobility analysis. PEF stages were treated as nominal categories, with the best category (i.e., Stage 1) serving as the reference group.
Cox regression models were subsequently used to estimate hazard ratios for the unadjusted and adjusted associations between the independent variable and each of the three outcomes. The independent variable included each PEF stage as compared with the reference stage. Potential confounders were age, gender, smoking status, body mass index (BMI), health status, chronic lung disease, number of other chronic conditions, cognitive status, and sampling design (to account for oversampling persons with slow gait speed). Whereas smoking status, chronic lung disease, and sampling design were forced into each multivariable model, the other potential confounders were included in the final models only if they met a forward selection criterion of p < 0.20.41,42 Higher order effects were tested for the continuous covariates (i.e., age and BMI) and included in the final models if they met the p < 0.20 criterion. Proportional hazards assumptions for the mortality model were tested by graphical means and by inclusion of interaction terms crossing the time-to-event outcome with each variable retained in the final multivariable model. If statistically significant at the 0.05 level, these interaction terms were retained in the final model. The proportional hazards assumption was not tested for the ADL and mobility disability outcomes, as these were measures of average rather than instantaneous risk (assessed over 2 and 6 months, respectively). Model goodness-of-fit was satisfied in all cases. SAS version 9.1.3 (SAS Institute; Cary, NC) was utilized for all analyses and a p-value < 0.05 (two-sided) was used to denote statistical significance.
RESULTS
As shown in Table 1, participants had a mean age of about 80 years, were largely female and white, and on average had a high school education and were not cognitively impaired. Smoking exposure was substantial, with nearly two out of every three participants being current or former smokers. The five most prevalent chronic conditions were hypertension, arthritis, diabetes, myocardial infarction, and chronic lung disease.
Table 1.
Baseline characteristics of study participants, N=753*
| Characteristic | Mean (SD) or No. (%) |
|---|---|
| Age in years | 78.4 (5.3) |
| Women | 487 (64.7) |
| Non-Hispanic white | 681 (90.4) |
| Education in years | 12.0 (2.9) |
| Smoking status | |
| Current | 63 (8.4) |
| Former | 416 (55.2) |
| Never | 274 (36.4) |
| MMSE score | 26.8 (2.5) |
| Fair-to-poor self-reported health | 211 (28.0) |
| Chronic lung disease | 131 (17.4) |
| Other chronic conditions | |
| Hypertension | 416 (55.3) |
| Arthritis | 227 (30.2) |
| Diabetes mellitus | 137 (18.2) |
| Myocardial infarction | 135 (17.9) |
| Cancer (other than minor skin cancers) | 124 (16.5) |
| Fracture other than hip since 50 years of age | 98 (13.0) |
| Stroke | 65 (8.6) |
| Congestive heart failure | 48 (6.4) |
One of the 754 participants did not achieve a peak flow (PEF) reading
SD= standard deviation; MMSE= Mini-Mental State Examination19
As shown in Table 2, measured PEF values were higher in ever-smokers than never-smokers. Relative to the predicted mean (adjusted for height, age, and gender), however, never smokers had a higher (i.e., less negative) PEF when expressed as a standardized residual. The 753 participants were somewhat evenly distributed across the five PEF stages, but never-smokers had the lowest proportion of participants in Stage 5. Nevertheless, regardless of smoking status, PEF values at below the 10th SR-percentile (Stage 5) were prevalent, ranging from 17.5% in never-smokers to 26.3% in ever-smokers.
Table 2.
Measured peak flow (PEF) for all participants, never-smokers, and ever-smokers
| PEF | All Participants N=753 |
Never-Smokers N=274 |
Ever-Smokers N=479 |
|---|---|---|---|
| Measured PEF in liters/minute* | 356 (122) | 344 (111) | 363 (127) |
| Mean (SD) | |||
| Measured PEF as a standardized residual† | −0.44 (1.30) | −0.28 (1.18) | −0.54 (1.35) |
| Mean (SD) | |||
| PEF Stage (SR-Percentile) | No. (%) | No. (%) | No. (%) |
| Stage 1 (80–100) | 120 (15.9) | 44 (16.1) | 76 (15.9) |
| Stage 2 (50–79) | 160 (21.3) | 72 (26.3) | 88 (18.4) |
| Stage 3 (30–49) | 127 (16.9) | 47 (17.2) | 80 (16.7) |
| Stage 4 (10–29) | 172 (22.8) | 63 (23.0) | 109 (22.8) |
| Stage 5 (< 10) ‡ | 174 (23.1) | 48 (17.5) | 126 (26.3) |
p= 0.04 for comparison of never-smokers versus ever-smokers.
p= 0.006 for comparison of never-smokers versus ever-smokers.
p= 0.006 for comparison of never-smokers versus ever-smokers, relative to all other stages combined.
SD= standard deviation; SR= standardized residuals.
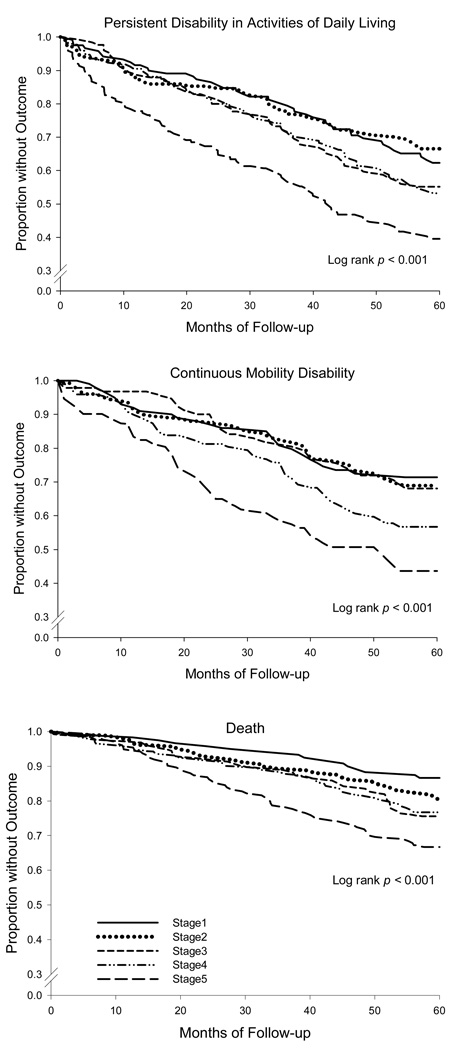
The incidence rates per 100 person-months (95% confidence intervals) were 1.00 (0.90, 1.12) for ADL disability, 0.80 (0.70, 0.93) for mobility disability, and 0.44 (0.38, 0.51) for death. Persistent ADL disability and continuous mobility disability developed in 313 (41.6%) and 195 (34.8%) participants, respectively; and 176 participants (23.4%) died, with nearly half (47.6%) of the deaths attributable to a cardiovascular cause. Figure 1 shows Kaplan-Meier curves for each of the three outcomes over the five-year study period stratified by PEF stage. A strong and statistically significant association was observed between PEF and the occurrence of each outcome, although there was some overlap in curves, which was most apparent for mobility disability (Stages 1–3) and persistent ADL disability (Stages 1–2 and 3–4). The outcome rates were particularly high for participants with the most severely diminished PEF (Stage 5).
Figure 1. Kaplan-Meier curves stratified by peak flow (PEF) stage.
For the analysis of disability in activities of daily living (ADL) and death, the number of participants at risk in stages 1 through 5 was 120, 160, 127, 172, and 174, respectively, for a total of 753. For the analysis of mobility disability, the number of participants at risk in stages 1 through 5 was 102, 129, 95, 122, and 112, respectively, for a total of 560. Statistical significance was determined by the log rank test.
Table 3 provides the hazard ratios for persistent ADL disability, continuous mobility disability, and death over the five-year study period according to PEF stage. In both the unadjusted and adjusted analyses, a graded relationship was generally observed between PEF and each outcome. At a PEF < 10th SR-percentile (Stage 5), which identified nearly a quarter of the cohort, the adjusted hazard ratios demonstrated clinically meaningful and statistically significant increases in the risk for each of the three outcomes.
Table 3.
Hazard ratios for disability and death over five years according to peak flow (PEF) stage*
| PEF Stage (SR-Percentile) |
Disability | Death | ||||
|---|---|---|---|---|---|---|
| Activities of Daily Living | Mobility † | |||||
| Unadjusted HR (CI) N=753 |
Adjusted HR (CI) N=753 |
Unadjusted HR (CI) N=560 |
Adjusted HR (CI) N=559 |
Unadjusted HR (CI) N=753 |
Adjusted HR (CI) N=752 |
|
| Stage 1 (80–100) |
1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Stage 2 (50–79) |
0.91 (0.60–1.37) |
0.92 (0.61, 1.39) |
1.08 (0.66–1.76) |
0.89 (0.54, 1.47) |
1.50 (0.82–2.75) |
1.71 (0.92, 3.15) |
| Stage 3 (30–49) |
1.28 (0.85–1.91) |
1.19 (0.79, 1.79) |
1.08 (0.64–1.83) |
0.98 (0.58, 1.68) |
1.94 (1.06–3.54) |
1.76 (0.96, 3.25) |
| Stage 4 (10–29) |
1.33 (0.91–1.94) |
1.24 (0.85, 1.82) |
1.63 (1.03–2.60) |
1.40 (0.86, 2.26) |
1.86 (1.04–3.32) |
1.68 (0.93, 3.02) |
| Stage 5 (< 10) |
2.13 (1.48–3.05) |
1.79 (1.23, 2.62) |
2.49 (1.58–3.93) |
1.89 (1.15, 3.10) |
2.89 (1.66–5.02) |
2.31 (1.29, 4.12) |
Stage 1 is the reference group. Each of the adjusted models included smoking status, chronic lung disease, and sampling design; other factors were included if they met the criteria described in the Statistical Analysis section. There was one missing BMI value in the adjusted mobility and mortality models.
Those with mobility disability at baseline were excluded.
SR=standardized residual; HR= hazard ratio; CI= 95% confidence interval.
DISCUSSION
In a large cohort of community-living persons aged ≥ 70 years, we found that PEF, when expressed as an SR-percentile, is associated with subsequent ADL disability, mobility disability, and death, independent of multiple potential confounders, including age, smoking, and chronic lung disease. These results suggest that PEF may be a potentially valuable risk assessment tool among community-living older persons.
In a cohort of older persons, the mechanisms that underlie a diminished PEF, and corresponding adverse outcomes, are likely to be multifactorial. One particularly important contributing factor is cigarette smoke, as evidenced in the current study in which two-thirds of the participants were either current or former smokers. Cigarette smoke is a leading cause of disability and death worldwide, largely due to cardiovascular disease, cancer, and chronic lung disease.21,26,27 Nearly one-fifth of our cohort reported chronic lung disease and about half of all deaths were attributable to cardiovascular disease.
Environmental factors independent of cigarette smoke can also contribute to a diminished PEF. Our participants resided in the greater New Haven area, which is ranked in the worst decile of all counties in the United States in terms of air pollution.28 In a cross-sectional sample of 3,912 adults who had never smoked, a 10-µg/m3 increase in daily nitrogen dioxide level, total suspended particulates, or ozone was associated with reductions in pulmonary function.29 Furthermore, regardless of smoking status, long-term exposure to particulate air pollution has been associated with increased cardiovascular events and death,30,31 with older persons being particularly vulnerable.32–34 Another possible contributing factor to a decreased PEF is asthma, which was included in our operational definition of chronic lung disease, and which may be also adversely affected by exposure to air pollution and tobacco.35
We found, however, that the risk of ADL disability, mobility disability, and death, although attenuated after adjustment for multiple confounders (including smoking and chronic lung disease) remained statistically significant at a PEF < 10th SR-percentile. This result suggests that other factors might contribute to the association between decreased PEF and adverse outcomes. In particular, because achieving a maximal PEF is effort-dependent,1 impairments in respiratory muscle strength, upper-extremity function (i.e., the PEF test requires that the participant handle the peak flow meter), cognitive status (i.e., the PEF test requires multiple steps), and mood may contribute to a diminished PEF, as well as to poor outcomes. Further research is needed to more completely evaluate the reasons why PEF exerts a detrimental effect.
Our study has several strengths. Missing data or attrition were minimal, and outcomes were monitored monthly over the course of five years. In addition, our use of the SR-percentile method for reporting PEF overcomes the limitations of prior studies that have investigated the association between PEF and adverse outcomes in older persons. The SR strategy effectively compares an individual’s measured PEF to the predicted mean of a normal reference population in accordance with published guidelines, and in a manner relevant to pulmonary function in an older population.1,9,14
We recognize that PEF has diagnostic limitations in terms of detecting airway obstruction, as reflected by its poor correlation with airway hyper-responsiveness and its limited sensitivity relative to the forced expiratory volume in one second (FEV1).36 Nevertheless, our results support the use of PEF in older persons not as a diagnostic measure of obstruction but, rather, as a risk assessment tool. PEF is simple to measure, inexpensive, readily available, and, when diminished, is associated with subsequent disability and death, independent of smoking status and chronic lung disease. In contrast, FEV1 measurements, even via a portable spirometer, are more expensive and require a respiratory maneuver that cannot be performed adequately in a large proportion of older persons who are frail but cognitively intact.37 In future studies, we plan to evaluate whether PEF or FEV1 is more strongly associated with adverse health and functional outcomes among community-living older persons.
Whether PEF provides useful prognostic information above and beyond other geriatric risk assessment tools, such as gait speed, is uncertain, but should be the focus of future research. Based on our results, however, PEF compares favorably with gait speed as a geriatric risk assessment tool. For example, in our fully adjusted model, a PEF cut point < 10th SR-percentile, identifying nearly a quarter of our cohort, was associated with an increased risk of ADL disability, mobility disability, and death of 79%, 89%, and 131%, respectively. In comparison, prior work has shown that gait speed, at a cut point < 1.0 meter/second, confers an increased risk of mobility disability and death of 120% and 64%, respectively.40
The results of the current study are applicable to clinical practice. Our elderly cohort is similar to those of other studies that evaluated older persons, in terms of smoking exposure and the prevalence of chronic lung disease.2,5,8,17,38,39 Nonetheless, the PEF thresholds documented in the current study for disability and death may differ for patient populations that have dissimilar exposures or clinical characteristics. The current study also focused on only a single PEF assessment. In future studies, we plan to evaluate changes in PEF over time and to determine whether these changes confer valuable prognostic information beyond that of a single assessment.
In conclusion, in a large cohort of community-living older persons, we found that a diminished PEF, expressed as an SR-percentile, is associated with subsequent disability and death, independent of multiple confounders, including age, smoking, and chronic lung disease. These results support the use of PEF as a potentially valuable risk assessment tool among older persons.
ACKNOWLEDGEMENTS
The authors thank Denise Shepard, Martha Oravetz, Shirley Hannan, Andrea Benjamin, Alice Kossack, Barbara Foster, Shari Lani, Alice Van Wie, and Amy Shelton for assistance with data collection; Wanda Carr and Geraldine Hawthorne for assistance with data entry and management; Peter Charpentier for development of the participant tracking system; Linda Leo-Summers for assisting with Figure 1; and Joanne McGloin for leadership and advice as the Project Director.
Financial Support:
The work for this report was funded by grants from the National Institute on Aging (NIA) (R37AG17560, R01AG022993). The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Dr. Fragoso was supported by a training grant from the NIA (T32AG1934) and is currently a recipient of an ASP/CHEST Foundation Geriatric Research Development Award. Dr. Gill is the recipient of an NIA Midcareer Investigator Award in Patient-Oriented Research (K24AG021507).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Quanjer PH, Lebowitz MD, Gregg I, et al. Peak expiratory flow: conclusions and recommendations of a working party of the European respiratory society. Eur Respir J. 1997;10 Suppl. 24:2S–8S. [PubMed] [Google Scholar]
- 2.Cook NR, Albert MS, Berkman LF, et al. Interrelationships of peak expiratory flow rate with physical and cognitive function in the elderly: MacArthur foundation studies of aging. J Gerontol Med Sci. 1995;50A:M317–M323. doi: 10.1093/gerona/50a.6.m317. [DOI] [PubMed] [Google Scholar]
- 3.Lan T-Y, Melzer D, Tom BDM, Guralnik JM. Performance tests and disability: Developing an objective index of mobility-related limitation in older populations. J Gerontol Med Sci. 2002;57:M294–M301. doi: 10.1093/gerona/57.5.m294. [DOI] [PubMed] [Google Scholar]
- 4.Schaub RT, Munzberg H, Borchelt M, et al. Ventilatory capacity and risk for dementia. J Gerontol Med Sci. 2000;55A:M677–M683. doi: 10.1093/gerona/55.11.m677. [DOI] [PubMed] [Google Scholar]
- 5.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate in an elderly population. Am J Epidemiol. 1989;130:66–78. doi: 10.1093/oxfordjournals.aje.a115324. [DOI] [PubMed] [Google Scholar]
- 6.Whitfield KE, Seeman TE, Miles TP, et al. Health indices as predictors of cognition among older African Americans: MacArthur studies of successful aging. Ethnicity Dis. 1997;7:127–136. [PubMed] [Google Scholar]
- 7.McCallum J, Simons LA, Simons J, Friedlander Y. Patterns and predictors of nursing home placement over 14 years: Dubbo study of elderly Australians. Australian J Ageing. 2005;24:169–173. [Google Scholar]
- 8.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate and 5-year mortality in an elderly population. Am J of Epidemiol. 1991;133:784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- 9.Quanjer Ph, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests European community for steel and coal. Official statement of the European respiratory society. Eur Respir J. 1993;6 S16:5–40. [PubMed] [Google Scholar]
- 10.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 12.Ferretti A, Giampiccolo, Cavalli, et al. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119:1401–1408. doi: 10.1378/chest.119.5.1401. [DOI] [PubMed] [Google Scholar]
- 13.Sahebjami H. Dyspnea in obese healthy men. Chest. 1998;114:1373–1377. doi: 10.1378/chest.114.5.1373. [DOI] [PubMed] [Google Scholar]
- 14.Fragoso CAV, Gahbauer EA, Van Ness PH, Gill TM. Reporting peak expiratory flow in older persons. J Gerontol Med Sci. 2007;62A:1147–1151. doi: 10.1093/gerona/62.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill TM, Desai MM, Gahbauer EA, et al. Restricted activity among community-living older persons: incidence, precipitants and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 16.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.National Asthma Education and Prevention Program Expert Panel Report 2: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Institutes of Health; 1997
- 21.Pauwels RA, Buist AS, Calverley PMA, et al. NHLBI/WHO Workshop Summary. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland ER, Cherniak RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2689–2697. doi: 10.1056/NEJMra030415. [DOI] [PubMed] [Google Scholar]
- 23.Sin DD, Jones RL, Mannino DM, Man SFP. Forced expiratory volume in 1 second and physical activity in the general population. Am J Med. 2004;117:270–273. doi: 10.1016/j.amjmed.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Gill TM, Allore HG, Holford TR, Guo Zhenchao. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;291:1596–1602. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 25.Gill TM, Allore HG, Hardy SE, Guo Z. The dynamic nature of mobility disability among older persons. J Am Ger Soc. 2006;54:248–254. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 26.Tager IB, Speizer FE. Risk estimates for chronic bronchitis in smokers: A study of male-female differences. Am Rev Respir Dis. 1976;113(5):619–625. doi: 10.1164/arrd.1976.113.5.619. [DOI] [PubMed] [Google Scholar]
- 27.CDC. Second National Report on Human Exposure to Environmental Chemicals: Tobacco Smoke. MMWR Morb Mortal Wkly Rep. 2002;51(14):300–303. [Google Scholar]
- 28. [Accessed March 4th];Environmental Defense Fund. 2007 Available at www.scorecard.org.
- 29.Schindler C, Kunzli N, Bongard JP, et al. Short-term variation in air pollution and in average lung function among never-smoker: The Swiss study on air pollution and lung diseases in adults. Am J Respir Crit Care Med. 2001;163:356–361. doi: 10.1164/ajrccm.163.2.9911116. [DOI] [PubMed] [Google Scholar]
- 30.Abbey DE, Nishino N, McDonnell WF, et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159:373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 31.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 32.Forastiere F, Stafoggia, Picciotto S, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. Am J Respir Crit Care Med. 2005;172:1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- 33.De Leon SF, Thurston GD, Ito K. Contribution of respiratory disease to nonrespiratory mortality associations with air pollution. Am J Respir Crit Care Med. 2003;167:1117–1123. doi: 10.1164/rccm.200205-409OC. [DOI] [PubMed] [Google Scholar]
- 34.Anderson HR, Spix C, Medina S, et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 european cities: Results from the APHEA project. Eur Respir J. 1997;10:1064–1071. doi: 10.1183/09031936.97.10051064. [DOI] [PubMed] [Google Scholar]
- 35.Tatum AJ, Shapiro GG. The effects of outdoor air pollution and tobacco smoke on asthma. Immunol Allergy Clin North Am. 2005;25:15–30. doi: 10.1016/j.iac.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Paggiaro PL, Moscato G, Giannini D, et al. Relationship between peak expiratory flow (PEF) and FEV1. Eur Respir J. 1997;10 Suppl. 24:39S–41S. [PubMed] [Google Scholar]
- 37.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age and Ageing. 2006;35:304–316. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 38.CDC. Cigarette smoking among adults—United States, 2004. Morb Mortal Wkly Rep. 2005;54(20):1121–1124. [PubMed] [Google Scholar]
- 39.Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22:268–273. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 40.Cesari M, Kritchevsky S, Penninx B, et al. Prognostic value of usual gait speed in well-functioning older people–results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 41.Concato J, Peduzzi P, Holford TR, Feinstein AR. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol. 1995;48:1495–1501. doi: 10.1016/0895-4356(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 42.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]