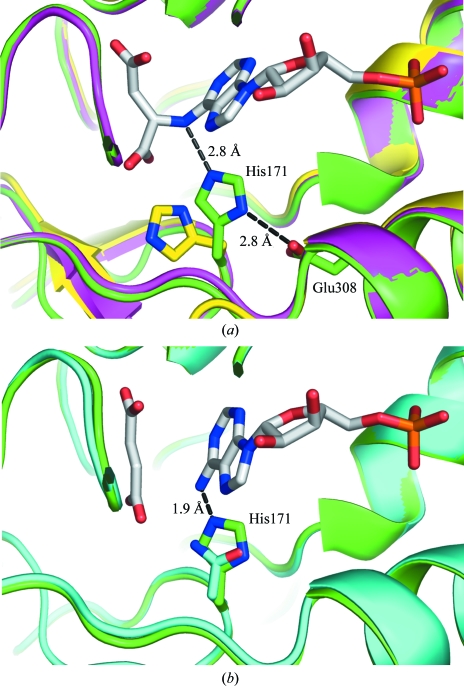
Figure 3.
Involvement of His171 in catalysis. (a) Overlay of the active sites of phosphate-bound (green), unliganded (yellow) and SAMP-bound H171A mutant (magenta) E. coli ASL. His171 of the phosphate-bound structure is perfectly positioned to donate a proton to the N6 atom of SAMP, supported by a charge-relay interaction with the side chain of Glu308. The side chains of His171 and Glu308 are shown and labelled. (b) Overlay of the phosphate-bound (green) and product-bound H171N mutant (PDB code 2ptq; cyan). The side chain of His171 in the phosphate-bound structure prevents the binding of AMP owing to a steric clash (1.9 Å) between the N6 atom of AMP and the histidine ring.