Abstract
The group of linkage potentials resulting from the energy of a physicochemical system expressed per mol of a reference component, say a polyfunctional macromolecule, leads to the concept of binding capacity. This concept applies equally to both chemical and physical ligands and opens the way to consideration of higher-order linkage relationships. It provides a means of exploring the consequences of thermodynamic stability on generalized binding phenomena in biopolymers.
Full text
PDF

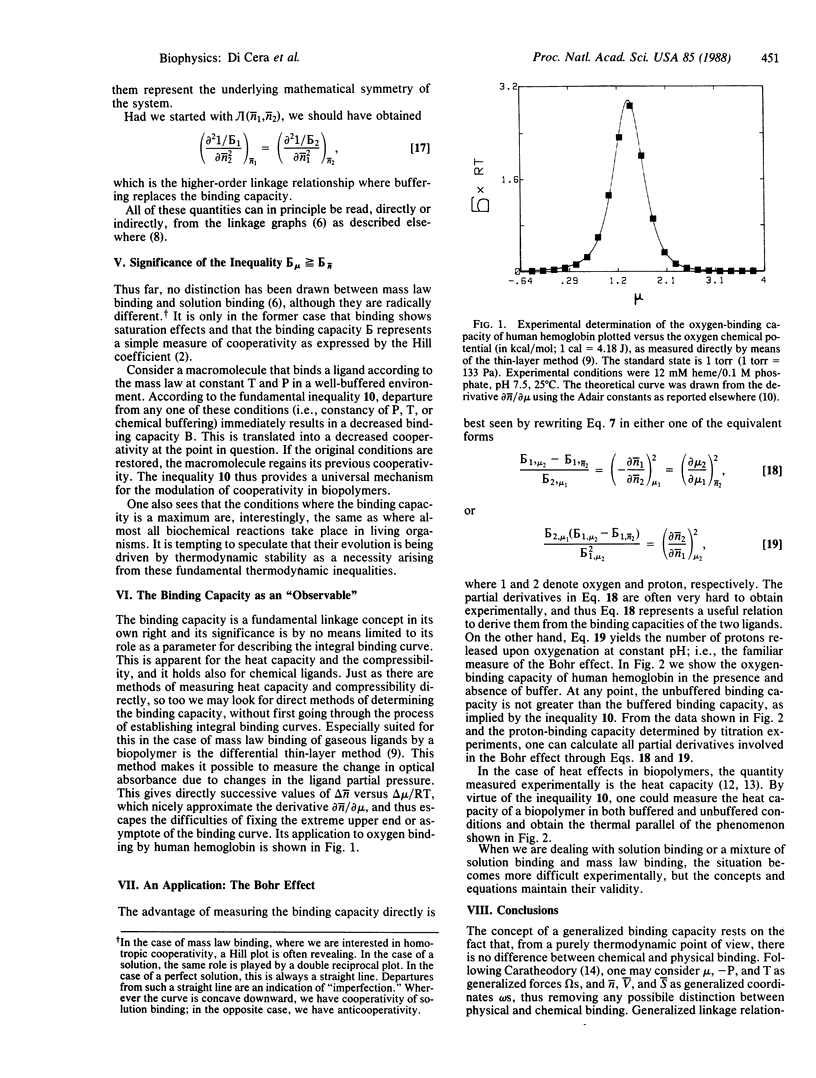
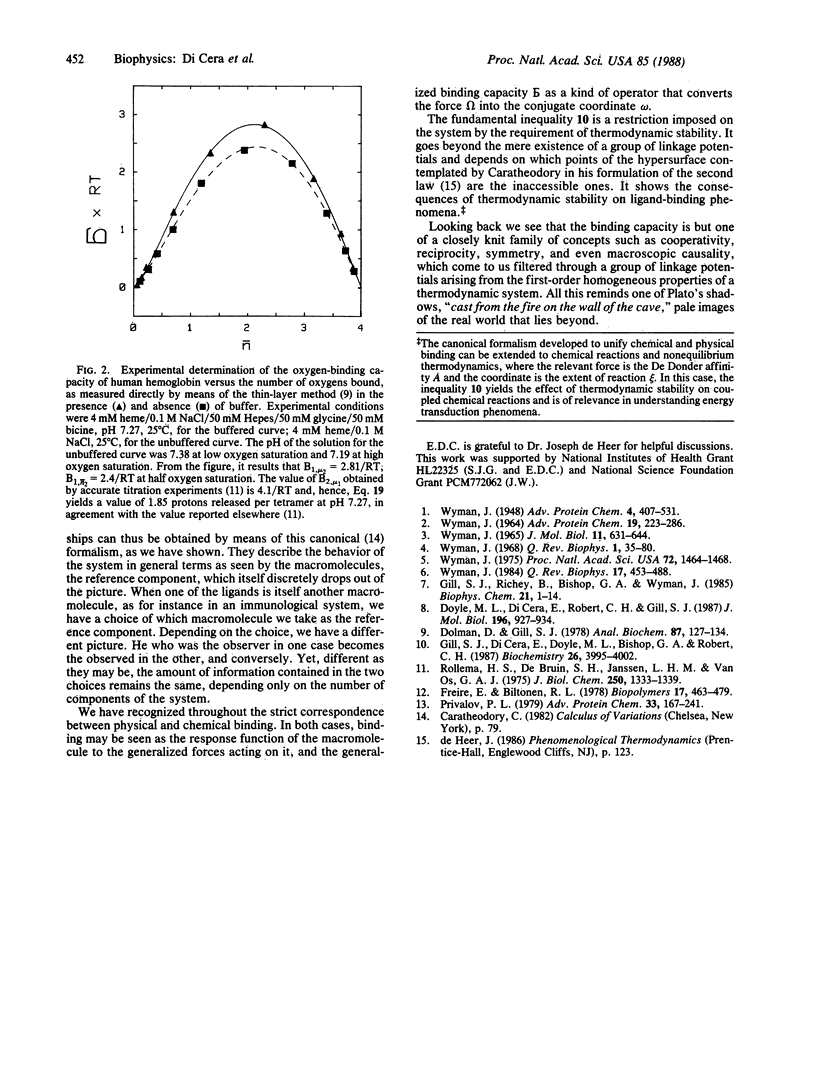
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dolman D., Gill S. J. Membrane-covered thin-layer optical cell for gas-reaction studies of hemoglobin. Anal Biochem. 1978 Jun 15;87(1):127–134. doi: 10.1016/0003-2697(78)90576-6. [DOI] [PubMed] [Google Scholar]
- Doyle M. L., Di Cera E., Robert C. H., Gill S. J. Carbon dioxide and oxygen linkage in human hemoglobin tetramers. J Mol Biol. 1987 Aug 20;196(4):927–934. doi: 10.1016/0022-2836(87)90414-1. [DOI] [PubMed] [Google Scholar]
- Gill S. J., Di Cera E., Doyle M. L., Bishop G. A., Robert C. H. Oxygen binding constants for human hemoglobin tetramers. Biochemistry. 1987 Jun 30;26(13):3995–4002. doi: 10.1021/bi00387a038. [DOI] [PubMed] [Google Scholar]
- Gill S. J., Richey B., Bishop G., Wyman J. Generalized binding phenomena in an allosteric macromolecule. Biophys Chem. 1985 Jan;21(1):1–14. doi: 10.1016/0301-4622(85)85001-8. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Rollema H. S., de Bruin S. H., Janssen L. H., van Os G. A. The effect of potassium chloride on the Bohr effect of human hemoglobin. J Biol Chem. 1975 Feb 25;250(4):1333–1339. [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- WYMAN J. THE BINDING POTENTIAL, A NEGLECTED LINKAGE CONCEPT. J Mol Biol. 1965 Mar;11:631–644. doi: 10.1016/s0022-2836(65)80017-1. [DOI] [PubMed] [Google Scholar]
- Wyman J. A group of thermodynamic potentials applicable to ligand binding by a polyfunctional macromolecule. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1464–1468. doi: 10.1073/pnas.72.4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman J. Linkage graphs: a study in the thermodynamics of macromolecules. Q Rev Biophys. 1984 Nov;17(4):453–488. doi: 10.1017/s0033583500004881. [DOI] [PubMed] [Google Scholar]
- Wyman J. Regulation in macromolecules as illustrated by haemoglobin. Q Rev Biophys. 1968 May;1(1):35–80. doi: 10.1017/s0033583500000457. [DOI] [PubMed] [Google Scholar]


