Abstract
Autoimmune regulator (Aire) has been viewed as a central player in the induction of tolerance. This study examines, whether Aire can modulate the production of the thymic chemokines involved in cortico-medullary migration and thus play a role in intrathymic thymocyte migration and maturation.
Aire deficiency resulted in reduced gene expression and protein levels of the CCR4 and CCR7 ligands in whole thymi of mice, as determined by quantitative PCR analysis and ELISA. The expression of the CCR4 ligands coincided with Aire expression in the CD80high medullary epithelial cells (mTECs), whereas the expression of the CCR7 ligands was detected in other cell populations. Also, the expression pattern of the CCR4 and CCR7 ligands follows that of Aire during postnatal but not during embryonic development. In vitro, overexpression of Aire resulted in an upregulation of selected CCR4 and CCR7 ligands, which induced selective migration of double-positive (DP) and single positive (SP) CD4+ cells. In vivo, Aire deficiency resulted in a diminished emigration of mature CD4+ T-cells from the thymi of 5-day-old mice
In conclusion, Aire regulates the production of CCR4 and CCR7 ligands in mTECs and alters the coordinated maturation and migration of thymocytes. These results suggest a novel mechanism behind the Aire-dependent induction of central tolerance.
Keywords: thymus, chemokines, autoimmunity
Introduction
The thymus is the primary lymphoid organ involved in the development of thymocytes and also has an essential role in establishing immune tolerance (1). Impaired clonal deletion or ineffective functional inactivation of self-reactive T-cells in the thymic medulla can lead to a breakdown of central tolerance and the development of autoimmune diseases.
An essential role in the regulation of central tolerance has been attributed to the Autoimmune regulator (Aire)3 (2). Aire is predominantly expressed in medullary thymic epithelial cells (mTECs) (3) and has several structural features, such as SAND and PHD finger domains, that are characteristic of proteins involved in transcriptional control (2, 4). In humans, mutations in AIRE can cause autoimmune-polyendocrinopathy-candidiasis ectodermal dystrophy (APECED), a syndrome characterized by the presence of antibodies to multiple self antigens and lymphocytic infiltration of endocrine glands, which leads to endocrine autoimmune disorders (5, 6). Similarly, in mice, Aire deficiency results in the development of a variety of autoantibodies and lymphocytic infiltration of multiple tissues (7). In addition, it has been demonstrated that Aire-deficient mice fail to delete organ-specific lymphocytes, thus indicating a defect in negative selection (8).
Aire has been suggested to induce central tolerance by regulating tissue-specific antigen (TSA) expression in the mTECs. mTECs can express thousands of TSAs to the developing thymocytes (9, 10), leading to deletion of self-reactive T-cells and, indeed, the purified mTEC population from Aire-deficient mice shows a decreased expression of many (but not all) TSAs (7). However, there is also evidence that impaired expression of TSAs is not the only mechanism behind Aire-induced autoimmunity. For example, Aire deficient mice have been shown to develop a Sjögren’s syndrome-like autoimmune reaction to α-fodrin, a self antigen not regulated by Aire (11). Similarly, Aire-deficient NOD mice have been shown to develop autoimmune pancreatitis to isomerase A2, another Aire-independent self antigen (12). Therefore, it is likely that Aire has an additional effect on negative selection independent of its effect on TSA expression (13, 14). The precise mechanism, however, has not yet been characterized.
Thymocyte development, including negative selection, is also dependent on their organized migration through distinct thymic niches, which promotes timely interactions with cortical and medullary thymic epithelial cells (cTEC and mTEC, respectively) and with medullary dendritic cells (mDCs) (reviewed in (15)). The organized migration of thymocytes is orchestrated by the expression of a variety of chemokines in different compartments of the thymic stroma and by stepwise expression of respective chemokine receptors on the developing thymocytes. Thus, the ligands for the chemokine receptors CCR9 and CXCR4 are responsible for the outward relocation of thymocytes mediating the migration of CD4−CD8− double negative (DN) thymocytes from the cortico-medullary junction to the subcapsular area, where they are positively selected (15). The CD4+CD8+ double positive (DP) thymocytes are then guided through the cortex to the medulla. This cortico-medullary migration depends on the expression of two chemokine receptors, CCR4 and CCR7 and their corresponding ligands (15). CCR4 and CCR7 are predominantly expressed on DP and single positive (SP) CD4+ thymocytes (16-18), while the ligands for CCR4 and CCR7 are produced predominantly in the thymic medulla (16, 17, 19-21). Also, the lack of CCR7 has been shown to result in delayed emigration of mature thymocytes (20), suggesting an impaired cortico-medullary migration. Since the medulla is believed to be the site of negative selection, the coordinated expression of CCR4 and CCR7 and their corresponding ligands is likely to direct the induction of central tolerance (15). Interestingly, array data from Aire KO mice suggests that in addition to down-regulation of TSAs, there is also an aberrant expression of a number of chemokines in the mTEC population (7, 22). Whether Aire itself can regulate the expression of chemokines or has any impact on thymocyte migration has not yet been characterized.
This study aims to clarify whether Aire can regulate thymic chemokine expression and affect the migration of thymocytes. Since CCR4 and CCR7 have been shown to participate in cortico-medullary migration and negative selection, we characterize the expression of three ligands for CCR4 (CCL5, CCL17 and CCL22), and two ligands for CCR7 (CCL19 and CCL21). In comparison, we also measure the expression of a chemokine known to mediate the outward relocation of DN thymocytes, the CCR9 ligand CCL25 (15). We ascertain that Aire deficiency results in reduced gene expression and protein levels of both the CCR4 and CCR7 ligands. We show that whereas the CCR4 ligands are induced in the same cell population as Aire, the CCR7 ligands are produced by the adjacent cells. We provide evidence that postnatally, the expression pattern of Aire closely follows that of CCR4 and CCR7 ligands and that overexpression of Aire can induce the expression of thymic chemokines. Finally, we show that overexpression of Aire results in an increased migration of DP and CD4+ thymocytes, whereas lack of Aire results in a delay in mature CD4+ cell emigration.
Material and Methods
Mice and cell cultures
C57BL/6 mice deficient for Aire gene were generated at The Walter and Eliza Hall Institute (Melbourne, Australia) via homologous recombination of targeting vectors in mouse C57BL/6 embryonic stem (ES) cells (23). Insertion of the Aire targeting vector disrupted exon 8 and brought the LacZ reporter gene under the control of the endogenous Aire promoter, creating an Aire-LacZ fusion. A phosphoglycerate kinase neomycin (PGK-Neo) cassette was used to select positive recombination events and was later removed using the flanking LoxP sites and Cre recombinase. The CCR7 deficient C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). All mice were bred and maintained at the mouse facility of the Institute of Molecular and Cell Biology, Tartu University.
Thymic epithelial 1C6 cell line (24) was kindly provided by G. Holländer (University of Basel, Switzerland) and was cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (Gibco BRL).
Chemokine expression in whole tissues by real-time quantitative PCR (qPCR)
Thymi and inguinal lymph nodes were dissected from 6 to 8-week-old WT, Aire-Het (heterozygote) and Aire KO mice. RNA was isolated using TRIzol (Invitrogen, Life Technologies) and reverse-transcribed to cDNA using the SuperScript™ III Reverse Transcriptase (Invitrogen, Life Technologies). qPCR was performed with the ABI Prism 7900 SDS instrument (Applied Biosystems) using qPCR SYBR Green Core Kit (Eurogentec) according to the manufacturer’s instructions. The amplification program included an initial denaturation step at 95 °C for 10 min, followed by denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 1 min, for 40 cycles. SYBR Green fluorescence was measured after each extension step, and the specificity of amplification was evaluated by melting curve analysis. Every sample was run in three parallel reactions. Primers used to amplify specific gene products from murine cDNA were beta-2-microglobulin sense, 5′-tgagactgatacatacgcctgca-3′; beta-2-microglobulin antisense, 5′-gatgcttgatcacatgtctcgatc-3′; K2–8 sense, 5′-aggagctcattccgtagctg-3′; K2–8 antisense, 5′-tctgggatgcagaacatgag-3′; Aire sense, 5′-tcctcaatgagcactcatttgac-3′; Aire antisense, 5′-ccacctgtcatcaggaagag-3′; CCL-5 sense, 5′-gtgcccacgtcaaggagtat-3′; CCL-5 antisense, 5′-cccacttcttctctgggttg-3′; CCL-17 sense, 5′-agtggagtgttccagggatg-3′; CCL-17 antisense, 5′-ccaatctgatggccttcttc-3′; CCL-22 sense, 5′-ctgatgcaggtccctatggt-3′; CCL-22 antisense, 5′-ggagtagcttcttcacccag-3′; CCL-19 sense, 5′-ctgcctcagattatctgccat-3′; CCL-19 antisense, 5′-cttccgcatcattagcaccc-3′; CCL-21 sense, 5′-ccctggacccaaggcagt-3′, CCL-21 antisense, 5′-aggcttagagtgcttccggg-3′; CCL-25 sense, 5′-gtgctgtgagattctacttcc-3′; CCL-25 antisense, 5′-tatggtttgacttcttcctttcag-3′. The relative gene expression levels were calculated using the comparative Ct (ΔΔCt) method (according to Applied Biosystems), where the relative expression is calculated as 2−ΔΔCt, and where Ct represents the threshold cycle. Except for the analysis of Aire and chemokines in developmental dynamics (see below) the data was normalized to the expression level of beta-2-microglobulin gene.
Chemokine protein levels in whole thymi by ELISA
Thymi from WT or Aire KO mice were dissected and collected into 600μl of PBS with 1:1000 of Protease Inhibitor Cocktail (Sigma-Aldrich). The tissues were homogenized using gentleMACS™ Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) as recommended by the manufacturer, and the CCL5, CCL17, CCL22, CCL19 and CCL21 levels were measured in the homogenates by ELISA (all from R&D Systems).
Cell sorting
Thymi from 6–8 week old WT or Aire KO mice were dissected and collected into RPMI. Small cuts were made into the capsules of thymi and the thymocytes were released by repetitive pipeting. The remaining thymic fragments were incubated in 0.5 mg/ml dispase/collagenase (Roche) and 5 μg/ml DNase I (AppliChem) in PBS at 37 °C for 20 min, with gentle agitation. The released cells were collected to separate fractions and fresh enzyme solution was added 4 times. Each cell fraction was counted and the final two or three digested fractions pooled. A negative depletion was performed to enrich for CD45-cells using CD45 microbeads (Miltenyi Biotec) and the AutoMACS system (Miltenyi Biotec), as per the manufacturer’s instructions. The negative fraction was stained with anti-G8.8-FITC (anti-EpCAM, generated from a G8.8 hybridoma cell line), anti-Ly51-PE (BD Biosciences), anti-CD45-PerCP-Cy5.5 (BD Biosciences) and anti-CD80 biotin (BD Biosciences) followed by second-stage staining with Streptavidin-PE-Cy7 (Serotec Ltd, Oxford, UK). Cell sorting and analysis was performed on a FACSAria (BD Biosciences) instrument to get the fractions of mTECs (CD45−, G8.8high, Ly51low) and cTECs (CD45−, G8.8low, Ly51high). According to the CD80 expression the mTEC fraction was further divided into the CD80high and CD80low mTECs. Thereafter, the RNA was purified by using Rneasy Micro Kit (Qiagen), followed by reverse-transcription and qPCR as described above.
Developmental dynamics
Embryonic (E13.5, E15.5 and E17.5), newborn (NB), neonatal day 10 (10d) and adult (6-week, 6-month) WT mouse thymi were dissected and used in developmental dynamics analysis. RNA was isolated using TRIzol, followed by reverse-transcription and qPCR as described above. In order to overcome variations in the cellular composition during development and to detect the expression of Aire and chemokines in the epithelial cell population only, the data was normalized to the expression level of keratin 8 (K8) mRNA, which is expressed selectively by thymic epithelial cells, shows equal expression level in the mTECs and cTECs, and is not influenced by Aire gene expression (7, 22).
Adenoviral infection
An adenoviral expression system (AdAire-GFP vs Ad-GFP) was used as previously described (25). TEC 1C6 cells were infected at approximately 70% confluence with Ad-Aire-GFP or Ad-GFP in 500 μl serum-free OptiMEM (containing 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B) for 1h. The medium was replaced thereafter with standard medium, and the cells were further incubated for 48 hours. The cells were then collected using Trypsin-EDTA and assessed for infection rate by quantifying the percentage of GFP positive cells in Ad-Aire-GFP or Ad-GFP infected TECs by FACSCalibur (BD Biosciences). RNA was isolated from the cells using TRIzol, followed by reverse-transcription and qPCR as described above.
Thymocyte chemotaxis in vitro
Thymocyte chemotaxis was assayed in Transwell inserts (Corning, NY) with a 5-μm-pore diameter. Thymocytes were obtained from thymi of 6–8 week old WT C57BL/6 mice. Small cuts were made into the capsules of thymi and the thymocytes were released by repetitive pipeting. The collected thymocytes were resuspended in standard TEC 1C6 medium at 5×107/ml and 100μl aliquots were loaded into the upper inserts. 600μl of supernatants from AdAire-GFP or Ad-GFP infected TEC 1C6 cells (see above) were used as chemotactic stimuli and were placed in the lower wells. As a positive control, 600μl of supernatant from TNF-α (10 ng/ml for 48 hours) stimulated 1C6 cells was used. After three hours of incubation, the migrated cells were counted and stained with anti-CD4-PE, anti-CD8a-FITC (both from Miltenyi Biotec), and anti-CD45-PerCP-Cy5.5 (BD Biosciences). The cells were analyzed with FACSCalibur (BD Biosciences) and the CD45+ cells further subdivided as DN (CD4−CD8−), SPCD4 (CD4+CD8−), SPCD8 (CD4−CD8+), and DP (CD4+CD8+) thymocytes.
In blocking experiments, the supernatants from Ad-Aire-GFP infected 1C6 cells were pre-incubated for 30 minutes at 37° C with the combination of antibodies against CCR4 ligands (α-CCL5, 10μg/ml; α-CCL17, 3μg/ml; α-CCL22, 3μg/ml, all from R&D Systems), with the combination of antibodies against CCR7 ligands (α-CCL19, 3μg/ml; α-CCL21, 0.5μg/ml, both from R&D Systems), or with the combination of antibodies against CCR4 plus CCR7 ligands. The effect of these supernatants was then tested against supernatant from Ad-Aire-GFP infected 1C6 cells pre-incubated with PBS (negative control) in chemotaxis assay (see above).
Emigration of mature T cells ex vivo and in vivo
The functional effect of Aire-deficiency on thymocyte maturation/emigration was studied using newborn thymus organ culture as previously described (20, 26), with some modifications. Whole thymi from 5-day old WT, Aire KO or CCR7 KO (positive control) mice were dissected and placed at the medium/air interface in the Transwell inserts (Corning, NY) with a 5-μm-pore diameter. The thymi were incubated at 37°C, 5% CO2 and, 16 hours later, the thymi as well as the emigrated cells were collected. The emigrated thymocytes as well as the thymocytes which remained in the thymus were counted and stained for CD45, CD4 and CD8 expression (see above). Mature thymocyte emigration was calculated as percent of emigration by taking the ratio of emigrated SP cells and remaining SP cells. In order to further characterize the emigration of mature thymocytes in 5-day old WT vs Aire KO mice, the cells were taken from both sides of the membrane (ie migrated vs unmigrated thymocytes) and co-stained with anti-CD45-APC, anti-TCRβ-Pe-Cy5 (both from BD Biosciences), anti-CD69-FITC, and anti-Qa2-PE (both from Abcam, Cambridge, UK). The emigration of CD4+TCRβ+CD69−Qa2+ cells was calculated as described above.
Also, the emigration of mature T cells was studied by counting the number of T-cells in spleens from 5-day old WT, Aire KO or CCR7 KO mice (20). The dissected spleens were digested with dispase/collagenase to get a single cell suspension. The cells were counted thereafter, and stained for CD45, CD4 and CD8 (see above). The number of mature, emigrated T-cells was calculated as the total number of SP cells per animal.
Results
Expression of Aire and thymic chemokines in thymi and lymph nodes
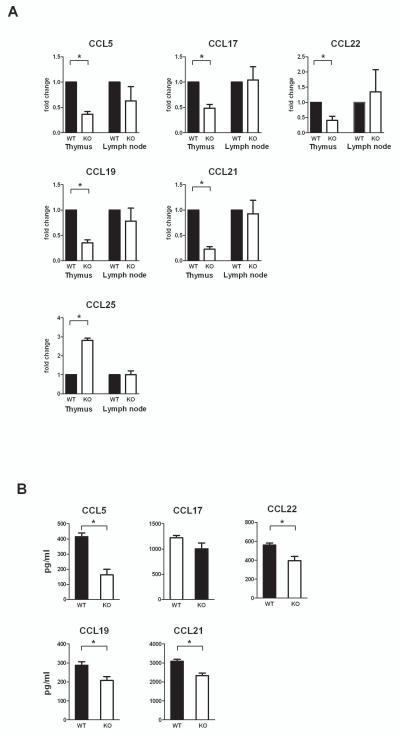
In order to determine whether Aire can affect thymic chemokine expression at the whole tissue level, we selected three previously characterized CCR4 ligands, CCL5, CCL17 and CCL22 and two previously characterized CCR7 ligands, CCL19 and CCL21 as well as the ligand for CCR9, CCL25 for investigation. We measured the expression level of these transcripts in whole thymi and lymph nodes from WT, Aire Het, and Aire KO mice. qPCR analysis showed a decreased expression of all CCR4 and CCR7 ligands in the thymi of Aire KO mice as compared to WT mice (Figure 1A). We did not, however, observe an allelic dose-dependency of the Aire gene for the CCR4 and CCR7 ligands as there was no significant difference between the WT and Aire Het mice (data not shown). In contrast to the CCR4 and CCR7, the expression of the CCR9 ligand CCL25, was increased in the Aire KO mice as compared with the WT mice as measured at the whole tissue level (Figure 1A).
Fig. 1.
(A) Chemokine expression in whole thymi and lymph nodes from wild type (WT) and Aire KO (KO) mice. Thymi and inguinal lymph nodes were dissected from six to eight weeks old mice and analyzed for chemokine expression by qPCR. The expression level of the CCR4 ligands, CCL5, CCL17, CCL22; and the CCR7 ligands, CCL19 and CCL21; was down-regulated in thymi from Aire deficient mice, whereas the expression of the CCR9 ligand, CCL25, showed an increased expression in the Aire KO thymi. The expression of the chemokines in whole lymph nodes was not changed. (B) Whole thymi from WT or Aire KO mice were homogenized and the protein levels measured by ELISA. The protein levels of CCL5, CCL22, CCL19 and CCL21 were significantly reduced in the Aire KO thymi. Data are mean with S.E.M. *, Student t-test, p<0.05; all other comparisons between WT and Aire KO are not significant, n=3–5.
On the contrary to the thymi, expression of the measured chemokines in lymph nodes was not affected by the expression level of Aire (Figure 1A).
In order to determine whether the changes in gene expression mirror the changes at protein level, we also measured the levels of CCR4 and CCR7 ligands in whole thymic tissues from WT and Aire KO mice by ELISA (Figure 1B). We found a significantly reduced levels of the CCR4 ligands CCL5 and CCL22, as well as the CCR7 ligands CCL19 and CCL21 in whole thymi from Aire KO mice as compared with the WT mice, whereas the level of CCL17, although slightly reduced in the Aire KO mice, was not significantly decreased (Figure 1B).
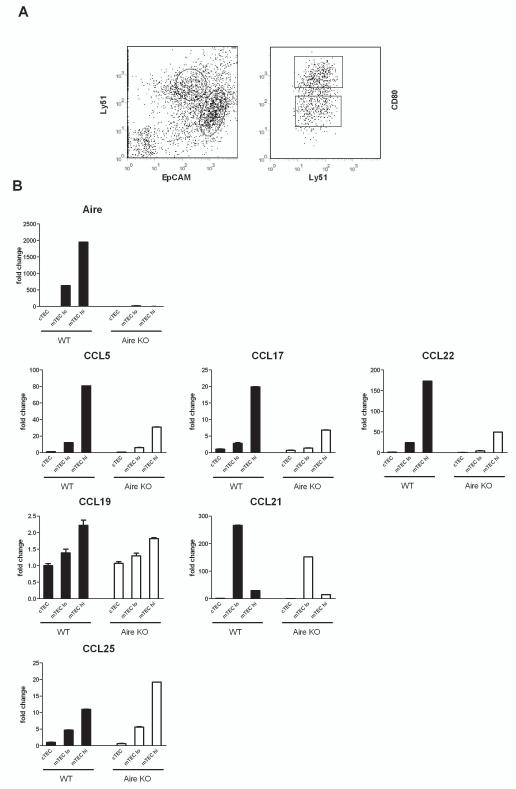
Coexpression of Aire and CCR4 ligands in CD80high mTECs
In order to determine whether the changes in chemokine expression reflect the changes in the same cell subpopulation in which Aire is expressed, we FACS-sorted the cortical and medullary epithelial cells and further divided the latter into the CD80low and CD80high subpopulations (Figure 2A). The expression levels of Aire, as well as the expression levels of all studied chemokines, were found to be remarkably higher in the mTEC population compared with the cTEC (Figure 2B). Within the mTECs the expression of Aire, as well as the expression of the CCR4 ligands CCL5, CCL17 and CCL22, was more prominent in the CD80high population of WT mice and downregulated in the corresponding subpopulation of the Aire KO mice. Regarding the CCR7 ligands, CCL19, although also showing the highest expression in the CD80high subpopulation of WT mTECs, was not significantly down-regulated in the same subpopulation from Aire KO mice, whereas CCL21, though downregulated in Aire KO mice, was preferentially expressed in the CD80low subpopulation of mTECs. The expression of the CCR9 ligand CCL25 was specifically upregulated in the CD80high mTECs of the Aire KO mice.
Fig. 2.
Thymic chemokine expression in cTEC, CD80low mTEC and CD80high mTEC population of thymi from wild type (WT) vs Aire KO mice. Thymi from 6–8 week old mice were enzyme digested and sorted by MACS and FACS, followed by expression analysis using qPCR. (A) Representative FACS profile indicating the subsets of cTEC (EpCAMlowLy51high) and mTEC (EpCAMhighLy51low). The mTECs were further sorted for CD80high and CD80low. (B) The expression of Aire as well as CCL5, CCL17, and CCL22 was predominantly localized in the CD80high mTECS of the WT mice and downregulated in corresponding population of the Aire KO mice. The expression of CCL19 showed the highest expression in the CD80high mTECs but was not significantly affected in the mTECs from Aire KO mice. The expression of CCL21 was localized preferentially in the CD80low mTECs and was down-regulated in the corresponding population of Aire KO mice. CCL25 was preferentially expressed in the CD80high mTECS and was upregulated in the corresponding population from Aire KO mice. Data are mean with S.E.M. of triplicate measurements of pooled samples from 10 mice per group.
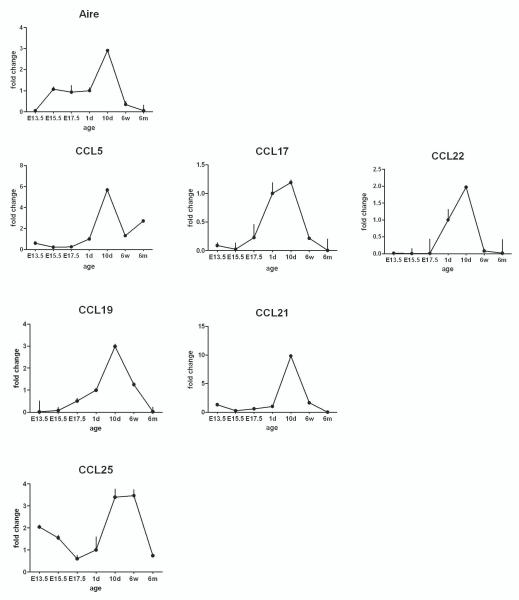
Expression of Aire and thymic chemokines in the ontogeny of WT mice
If the expression of thymic chemokines is dependent on Aire, this should be evident throughout the development of thymic tissue. However, the thymic cellular content and volume changes significantly during development. To limit our analysis to the epithelial cell subsets only, we normalized our data to the K8 gene, which is expressed independently of Aire by medullary and cortical epithelial cells. Thymi from various embryonic, neonatal, young or adult developmental stages were analyzed for Aire and thymic chemokine expression (Figure 3). Expression of the majority of chemokines, including CCL5, CCL17, CCL21 and CCL25 was already detectable at the very earliest time-point studied, E13.5, while Aire showed little if any expression at this point. After birth, however, the expression level of most of the thymic chemokines followed the pattern of Aire, peaking at day 10 and gradually decreasing thereafter. Again, the only exception was the CCR9 ligand, CCL25, whose expression started very high at E13.5, decreased gradually to E17.5, and reached a second peak at day 10, one which lasted until 6 weeks of age (Figure 3).
Fig. 3.
Aire and chemokine expression during ontogeny of normal thymus. Thymi were collected from wild type mice at indicated embryonic (E) or postnatal (day-d, week-w, month-m) time-points and the gene expression level analyzed by qPCR. Aire expression, as well as the expression of the CCR4 and CCR7 ligands reached their peak at 10d after birth followed by a gradual decrease. The expression of the CCR9 ligand, CCL25, showed two peaks one at E13.5 and the other between 10d and 6w of age. Data are mean with S.E.M. of triplicate measurements of one out of two representative experiments.
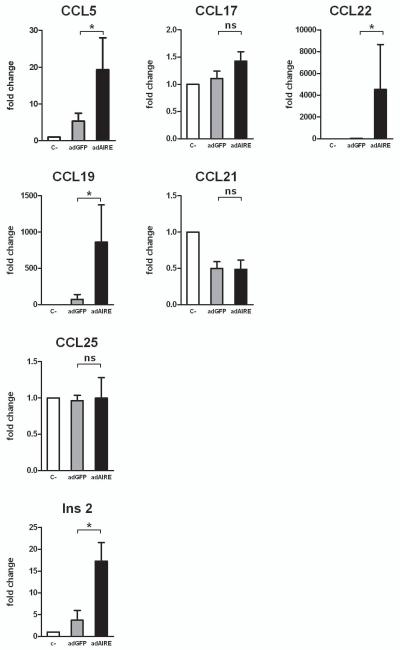
Overexpression of Aire increases thymic chemokine expression
Next we aimed to show that Aire, as a single factor, is sufficient to upregulate thymic chemokine expression. We used an adenoviral expression system (AdAire-GFP vs. Ad-GFP) to determine whether the specific overexpression of Aire has an effect on the expression of chemokines. Incubation with AdAire-GFP or Ad-GFP viruses resulted in a virtually equal infection rate as determined using FACS by evaluating the percentage of GFP positive cells at 48 hours (AIRE-GFP: 38.1±4.3% vs. ad-GFP: 42.8±2.8%; mean with S.E.M., n=3). However, as compared with Ad-GFP, the AdAire-GFP infection resulted in an increased expression of the CCR4 ligands CCL5 and CCL22; the CCR7 ligand CCL19; as well as the well-characterized Aire-regulated gene Insulin 2, whereas there was no change in the expression of CCL17, CCL21 or CCL25 (Figure 4). These results demonstrate that even in the absence of signals from other cell-types normally present in thymus, Aire expression is sufficient to induce the expression of a number of thymic chemokines in thymic epithelial cells.
Fig 4.
Over-expression of Aire induces chemokine expression in thymic epithelial cells. Thymic epithelial cell-line TEC 1C6 was infected with either negative control, Ad-GFP (adGFP) or Ad-Aire-GFP (adAIRE) and, 48 h later, the cells were harvested for RNA purification and qPCR analysis. Aire overexpression resulted in a significant increase in the expression of CCL5, CCL22 and CCL19, whereas there was virtually no change in the expression level of CCL17, CCL21 and CCL25. The well-characterized Aire-regulated gene, Insulin-2 (Ins-2) is also shown. Data are mean with S.E.M. *, Student t-test, p<0.05; ns= not significant, n=3–5.
Overexpression of Aire induces DP and SPCD4 thymocyte migration
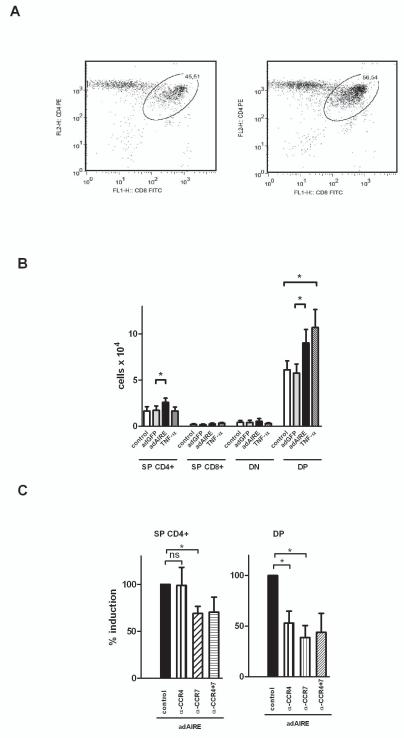
Since overexpression of Aire resulted in an upregulation of the CCR4 and CCR7 ligands, we wanted to clarify whether this overexpression can also result in the selective migration of thymocytes towards the Ad-Aire-GFP infected thymic epithelial cells. In this setting, infection with Ad-GFP did not have any effect on total numbers of thymocytes migrated (negative control: 0.084±0.01×106 vs. Ad.-GFP: 0.080±0.01×106; Student t-test, p>0.05, n=5), whereas overexpression of Aire resulted in a significant increase in migrated thymocyte numbers (Ad-Aire-GFP: 0.124±0.01×106, p<0.05 as compared with Ad-GFP). This increase in thymocyte migration was specific for the DP and SPCD4 cells, i.e. the cells expressing CCR4 and CCR7 (Figure 5A&B). In order to characterize the magnitude of the effect of Aire overexpression in this setting, we also used cell-supernatants from TNF-α stimulated thymic epithelial cells and evaluated the effect on thymocyte chemotaxis. Supernatants from TNF-α treated cells induced a significant increase in DP thymocyte chemotaxis, which was in the same order of magnitude as the effect induced by Ad-Aire-GFP. However, the supernatants from TNF-α treated cells had no effect on chemotaxis of other cell subsets (Figure 5B).
Fig.5.
Aire-induced CCR4 and CCR7 ligand expression results in increased chemotaxis of double-positive (DP) and single positive (SP) CD4+ thymocytes. Thymic epithelial cell-line TEC 1C6 was infected with either negative control, Ad-GFP (adGFP) or Ad-Aire-GFP (adAIRE) and, 48 h later, the cell supernatants were used as chemotactic stimuli for thymocytes in Transwell chemotaxis assay. Supernatants from TNF-α treated 1C6 were used as a positive control. The migrated cells were stained for CD4, CD8, and CD45 and analyzed by FACS. (A) Representative FACS plot of migrated thymocytes towards Ad-GFP vs Ad-Aire-GFP infected 1C6 cells. (B) Mean values with S.E.M of SP, double negative (DN), and DP thymocytes after migration towards the 1C6 cells infected with adGFP, adAIRE, negative control or TNF-α treated cells. Aire overexpression induced a selective migration of the DP and SPCD4 thymocytes whereas Ad-GFP alone did not have any significant effect. TNF-α treated supernatants induced migration of DP thymocytes but not other cell types. (C) Mean values with S.E.M. of SP CD4+ and DP thymocytes after migration towards adAIRE infected 1C6 cells pre-treated with antibodies against CCR4 ligands (α-CCR4), CCR7 ligands (α-CCR7) or the combination of CCR4 plus CCR7 ligands (α-CCR4+7). The Aire-induced migration of DP cells was inhibited by both the CCR4 and CCR7 ligand antibodies, whereas the SP CD4+ cell migration was inhibited by the CCR7 but not CCR4 ligand antibodies. *, Student t-test, p<0.05; ns= not significant, n=4–5.
Aire-induced thymocyte migration is inhibited by antibodies against CCR4 and CCR7 ligands
Consequently we aimed to clarify whether the effect of Aire overexpression on DP and SPCD4 chemotaxis is mediated by an increased release of CCR4 and CCR7 ligands. We used Ad-Aire-GFP infected cell supernatants together with combinations of antibodies to all known CCR4 and CCR7 ligands and characterized the migration of DP and SPCD4 thymocytes. The effect of Aire on SPCD4 migration was inhibited by the presence of antibodies against CCR7 but not CCR4 ligands (Figure 5C). However, the effect of Aire on DP cell migration was inhibited by both the CCR4 and the CCR7 ligand antibodies. The combination of CCR4 and CCR7 ligand antibodies did not produce any further effect when compared with CCR4 or CCR7 ligand antibodies alone (Figure 5C).
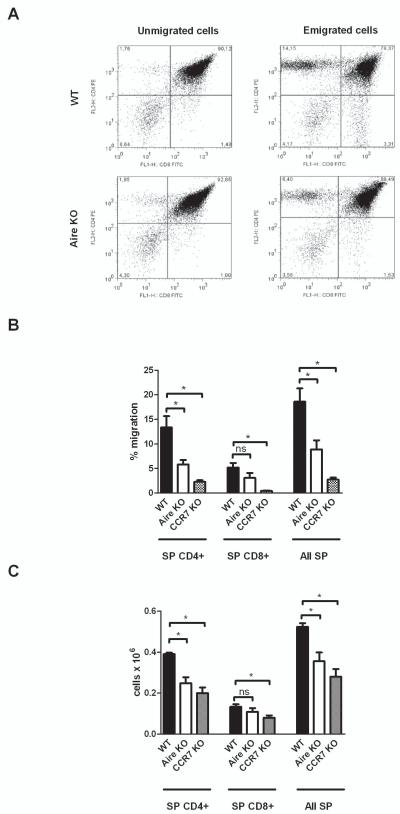
Aire deficiency results in delayed emigration of mature thymocytes
In order to determine whether the lack of Aire has also an impact on migration of thymocytes in vivo, we used a previously characterized model of thymocyte emigration that should reflect the cortico-medullary migration and maturation of early thymocytes. Using this model, it has been shown that in CCR7 KO mice, the impaired cortico-medullary migration of thymocytes results in delayed emigration of mature SP thymocytes, which can be detected only during the neonatal period (20). In this study, we used the thymi from 5-day old WT, Aire KO and CCR7 KO (positive control) mice and calculated the number of emigrated mature SP cells. We observed that the number of emigrated SPCD4 cells, as well as total number of SP cells, was significantly reduced in the 5-day old Aire KO thymi, as compared with WT thymi (Figure 6A and B). In the CCR7 KO mice, the numbers of emigrated SPCD4, SPCD8, as well as the total number of emigrated SP cells were all reduced as compared with the WT mice. In parallel, we characterized the maturation and emigration of early thymocytes by quantifying the number of mature T-cells in the spleen of WT, Aire KO and CCR7 KO mice at 5 days. We found that the number of both CD4+ and total T-cells was reduced in the Aire KO mice whereas in the CCR7 KO mice the numbers of CD4+, CD8+ as well as the total T-cells were all reduced (Figure 6C).
Fig 6.
Aire-deficiency results in delayed emigration of early mature thymocytes. Whole thymi from 5-day old wild type (WT), Aire KO or CCR7 KO mice were cultured on Transwell inserts for 16 hours, and stained for CD4, CD8, and CD45, followed by FACS analysis. In parallel, the cells from whole spleens of 5-day old mice were also stained and characterized. (A) Representative FACS plot of unmigrated and migrated thymocytes from WT and Aire KO mice. (B) Mean values with S.E.M. of single positive (SP) CD4+, SP CD8+, and all SP cells from WT, Aire KO or CCR7 KO thymi, calculated as percent of the total number of emigrated thymocytes. Aire deficiency resulted in reduced numbers of SP CD4+ cells, whereas CCR7 deficiency resulted in decreased numbers of SP CD4+ and SP CD8+ thymocytes. (C) Mean values with S.E.M. of total number of SP CD4+, SP CD8+, and all SP cells from spleens of WT, Aire KO or CCR7 KO mice. The number of CD4+ and the total number of SP cells was significantly reduced in the 5-day old Aire KO mice, whereas the CCR7 deficiency resulted in reduced numbers of all cell populations. *−p<0.05, Student t-test, n=5–6, ns= not significant.
Since Aire deficiency has been shown to result in reduced numbers of the SPCD4+ of the latest developmental stage (ie TCRβ+CD69−Qa2+) (27), we also aimed to characterize whether this difference can be detected in the emigration assay. However, by using thymi from 5-day old mice, we could not detect any significant difference in emigration of the CD4+TCRβ+CD69−Qa2+ thymocytes between the WT and Aire KO mice (% emigration mean±S.E.M; WT: 21.2±11.7, Aire KO: 17.3±4.7; Student t-test, p>0.05, n=5–6).
Discussion
Coordinated migration of thymocytes through distinct compartments of thymus is a prerequisite for controlled T-cell development. The migration of thymocytes from the cortico-medullary junction to the cortex and from the cortex to the medulla is coordinated on the one hand by stepwise expression of chemokine receptors on the thymocytes, and on the other hand by expression of different chemokines by different thymic cellular compartments (15). In this study, we show that in Aire-deficient mice there is a down-regulation of the two major groups of chemokines responsible for cortico-medullary migration: the ligands for CCR4 and CCR7. Our results thus point to a novel mechanism by which Aire may affect the induction of central tolerance. On the other hand, the expression of the chemokine known to mediate the outward relocation of DN thymocytes, CCL25 (28), is upregulated in the Aire KO mice, suggesting that the coordinated migration of thymocytes in Aire deficient mice may be influenced at a different level of maturation.
The effect of Aire deficiency on chemokine expression has been previously suggested by Anderson et al (8) using gene-chip analysis of mTECs from WT and Aire KO mice. In agreement with the previous report, the present study shows a reduction of CC17 and CCL22 as well as an increase in CCL25 in the Aire deficient mice. However, as opposed to the previous paper, we show also reduced levels of CCL5, CCL19 and CCL21 in the Aire KO thymi. These differences are likely to be due to the higher sensitivity of the q-PCR method, used in this study, or possibly due to the fact that whole thymi, rather than sorted mTECs were used.
In addition to the thymus, lymph nodes have recently been reported to express Aire, and it has been suggested that this peripheral Aire can also contribute to the induction of tolerance (29, 30). In this study, we show that at least at the whole tissue level, the majority of chemokines are not affected by Aire deficient lymph nodes. This finding, in agreement with a previous report, suggests that the number of target genes for Aire is different and more limited in the periphery (30) and also indicates a minor role for Aire in the regulation of peripheral chemokines.
The expression of Aire in the thymus is confined to a specific subset of epithelial cells, the CD80high mTECs (22). In this study, we show that the expression and regulation of all CCR4 ligands is indeed localized to the same subset of cells, suggesting that the effect of Aire on CCR4 ligand induction is located in, although not certainly limited to, the same cell population. The expression of the CCR7 ligands, on the other hand, is either not localized to the CD80high mTECs (CCL21) or is not regulated by Aire in this cell population (CCL19), which indicates an indirect effect of Aire on CCR7 ligand induction. Whereas the CD80low mTEC seems to be the target cell for CCL21 production, the source for CCL19 remains to be clarified. However, the likely candidates include thymic dendritic cells and fibroblasts, both of which are known to produce considerable amounts of the CCR7 ligands (31).
In order to determine whether the thymic expression of Aire and chemokines follow the same pattern during mouse development, we monitored their expression from E13.5 to 6 months. We detected a clear signal for many chemokines at E13.5, before the obvious increase in Aire signal at day E15.5, which suggests that in early development, mechanisms other than Aire trigger the chemokine expression needed for arrival of early thymocytes. However, during postnatal development and involution, both the CCR4 and the CCR7 ligands followed the expression pattern of Aire by peaking at day 10 and gradually declining thereafter. Thus, these data are compatible with Aire playing a role as a postnatal contributor to the regulation of chemokines behind cortico-medullary thymocyte migration.
We further characterized Aire-dependent chemokine expression by adenoviral experiments inducing Aire expression in thymic epithelial cells. We established that overexpression of Aire as a single factor is sufficient to induce the expression of the CCR4 ligands, CCL5 and CCL22, as well as the CCR7 ligand, CCL19. On the other hand, CCL17 and CCL21, although clearly Aire dependent in other experimental settings, were not induced, suggesting that optimal chemokine induction requires additional signals or cell-types. Likewise, the CCR9 ligand, CCL25, although negatively regulated in Aire deficient situation, was not reduced by Aire overexpression, suggesting again an indirect mechanism behind this effect.
As chemokine receptors are differentially expressed during different stages of thymocyte development, we also tested whether an increase in Aire-induced chemokine expression results in a significant and selective increase in thymocyte migration by using supernatants from cells overexpressing Aire. In this chemotaxis assay, we showed that induction of Aire can selectively induce migration of DP and SPCD4 thymocytes. The fact that these cell populations are the ones selectively expressing CCR4 and CCR7 and naturally migrating from cortex towards medulla (16-18), as well as the fact that the Aire-induced migration of these cells was inhibited by the presence of CCR4 and CCR7 ligand antibodies, further strengthens the idea that Aire is a contributor to the cortico-medullary migration of thymocytes.
Finally, we aimed to determine whether the lack of Aire results in significant changes to cortico-medullary migration of thymocytes in vivo. We used an experimental setting where the functional significance of CCR7 deficiency has previously been described (21). We showed that similar to the case in CCR7 KO mice, there is a delay of emigration of mature lymphocytes in the Aire KO mice. Interestingly, we see a significant delay of emigration for the CD4+ cells but not for the CD8+ cells, perhaps reflecting a predominant expression of CCR4 and CCR7 on SPCD4 thymocytes.
A delayed emigration of thymocytes is not the only similarity between the Aire deficient and CCR7 KO mice. Just like the Aire KO mice, CCR7 deficient mice are also characterized by lymphocytic infiltrations in multiple tissues, including the lachrymal gland, stomach, lung, salivary, pancreas and liver; they are also characterized by the presence of multiple autoantibodies, including the ones against α-fodrin (32). In addition, CCR7 deficient mice are characterized by altered thymic micro-architecture, illustrated by smaller but more numerous medullary areas, which are sometimes misplaced under the cortical rim (33). Interestingly, recent publications have also shown an altered differentiation program in the mTECs of Aire KO mice (34, 35), further stressing the similarities between Aire deficient and CCR7 deficient mice. Together, these similarities suggest the involvement of a common mechanism for the ligands of CCR7 and Aire.
Also, a recent study has shown a severe reduction of CD80high mTECs in H2-Aa_/_ mice (36), indicating a requirement for SPCD4 thymocytes in thymic medulla maturation and proliferation. It remains to be determined whether the impaired cortico-medullary migration of thymocytes or delayed maturation of SPCD4 cells described in our study contributes to the postponed functional maturation of thymic medulla or changes in mTEC differentiation. Another study has provided evidence that the final maturation of SP thymocytes might be blocked in the Aire KO mice (27). However, we do not see any difference in early emigration of CD4+TCRβ+CD69−Qa2+ cells between Aire KO and WT mice suggesting that mechanisms other than impaired cortico-medullary migration might mediate this effect.
In conclusion, we show that Aire has a dose-dependent effect on CCR4 and CCR7 ligand expression in the thymus. The CCR4 ligands are regulated by Aire in the CD80high mTECs, whereas the CCR7 ligands are upregulated in adjacent cells. Overexpression of Aire as a single factor induces the expression of CCL5, CCL22 and CCL19 and results in an increase in DP and SPCD4 thymocyte migration, an effect inhibited by the CCR4 and CCR7 ligand antibodies. Also, the lack of Aire in vivo results in a delayed emigration of mature thymocytes. These data suggest that regulation of thymic chemokines and cortico-medullary migration of thymocytes contribute to the Aire-dependent induction of central tolerance.
Acknowledgements
We thank all past and present lab and support staff in the labs of H.S.S. and P.P.
Footnotes
This work was supported by Eurothymaide and EURAPS, 6th FP of the EU (P.P. and H.S.S.); the Wellcome Trust Senior fellowship grant (P.P.); NHMRC fellowships 171601 and 461204, NHMRC program grants 257501, 264573 and 406700 (H.S.S); Estonian Science Foundation Grants 7559, 7197, 6663 (M.L, K.K, P.P); European Regional Fund and Archimedes Foundation (M.L, K.K, P.P); and Estonian targeted financiation grant SF0180021s07 (P.P.)
- Aire
- Autoimmune regulator
- mTEC
- medullary thymic epithelial cell
- cTEC
- cortical thymic epithelial cell
- DN
- double negative
- DP
- double positive
- SP
- single positive
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunological reviews. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 2.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nature genetics. 1997;17:393–398. [Google Scholar]
- 3.Heino M, Peterson P, Kudoh J, Nagamine K, Lagerstedt A, Ovod V, Ranki A, Rantala I, Nieminen M, Tuukkanen J, Scott HS, Antonarakis SE, Shimizu N, Krohn K. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochemical and biophysical research communications. 1999;257:821–825. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 4.Gibson TJ, Ramu C, Gemund C, Aasland R. The APECED polyglandular autoimmune syndrome protein, AIRE-1, contains the SAND domain and is probably a transcription factor. Trends in biochemical sciences. 1998;23:242–244. doi: 10.1016/s0968-0004(98)01231-6. [DOI] [PubMed] [Google Scholar]
- 5.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. The Journal of clinical endocrinology and metabolism. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 6.Peterson P, Peltonen L. Autoimmune polyendocrinopathy syndrome type 1 (APS1) and AIRE gene: new views on molecular basis of autoimmunity. Journal of autoimmunity. 2005;25(Suppl):49–55. doi: 10.1016/j.jaut.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science (New York, N.Y. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nature immunology. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 10.Gillard GO, Farr AG. Features of medullary thymic epithelium implicate postnatal development in maintaining epithelial heterogeneity and tissue-restricted antigen expression. J Immunol. 2006;176:5815–5824. doi: 10.4049/jimmunol.176.10.5815. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, Sakaguchi S, Ueno T, Takahama Y, Uchida D, Sun S, Kajiura F, Mouri Y, Han H, Matsushima A, Yamada G, Matsumoto M. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 12.Niki S, Oshikawa K, Mouri Y, Hirota F, Matsushima A, Yano M, Han H, Bando Y, Izumi K, Matsumoto M, Nakayama KI, Kuroda N, Matsumoto M. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. The Journal of clinical investigation. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathis D, Benoist C. A decade of AIRE. Nature reviews. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 14.Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nature reviews. 2008;8:948–957. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nature reviews. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 16.Chantry D, Romagnani P, Raport CJ, Wood CL, Epp A, Romagnani S, Gray PW. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood. 1999;94:1890–1898. [PubMed] [Google Scholar]
- 17.Annunziato F, Romagnani P, Cosmi L, Beltrame C, Steiner BH, Lazzeri E, Raport CJ, Galli G, Manetti R, Mavilia C, Vanini V, Chantry D, Maggi E, Romagnani S. Macrophage-derived chemokine and EBI1-ligand chemokine attract human thymocytes in different stage of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J Immunol. 2000;165:238–246. doi: 10.4049/jimmunol.165.1.238. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JJ, Pan J, Butcher EC. Cutting edge: developmental switches in chemokine responses during T cell maturation. J Immunol. 1999;163:2353–2357. [PubMed] [Google Scholar]
- 19.Alferink J, Lieberam I, Reindl W, Behrens A, Weiss S, Huser N, Gerauer K, Ross R, Reske-Kunz AB, Ahmad-Nejad P, Wagner H, Forster I. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. The Journal of experimental medicine. 2003;197:585–599. doi: 10.1084/jem.20021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. The Journal of experimental medicine. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueno T, Hara K, Willis MS, Malin MA, Hopken UE, Gray DH, Matsushima K, Lipp M, Springer TA, Boyd RL, Yoshie O, Takahama Y. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 22.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. The Journal of experimental medicine. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubert FX, Kinkel SA, Webster KE, Cannon P, Crewther PE, Proeitto AI, Wu L, Heath WR, Scott HS. A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 24.Mizuochi T, Kasai M, Kokuho T, Kakiuchi T, Hirokawa K. Medullary but not cortical thymic epithelial cells present soluble antigens to helper T cells. The Journal of experimental medicine. 1992;175:1601–1605. doi: 10.1084/jem.175.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kont V, Laan M, Kisand K, Merits A, Scott HS, Peterson P. Modulation of Aire regulates the expression of tissue-restricted antigens. Molecular immunology. 2008;45:25–33. doi: 10.1016/j.molimm.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CK, Kim K, Welniak LA, Murphy WJ, Muegge K, Durum SK. Thymic emigrants isolated by a new method possess unique phenotypic and functional properties. Blood. 2001;97:1360–1369. doi: 10.1182/blood.v97.5.1360. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Li Y, Yao JY, Jin R, Zhu MZ, Qian XP, Zhang J, Fu YX, Wu L, Zhang Y, Chen WF. Developmental pathway of CD4+CD8− medullary thymocytes during mouse ontogeny and its defect in Aire−/−mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18175–18180. doi: 10.1073/pnas.0708884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. European journal of immunology. 2004;34:3652–3663. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nature immunology. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 30.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science (New York, N.Y. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nature reviews. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 32.Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, Forster R. Generalized multi-organ autoimmunity in CCR7-deficient mice. European journal of immunology. 2007;37:613–622. doi: 10.1002/eji.200636656. [DOI] [PubMed] [Google Scholar]
- 33.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. The Journal of experimental medicine. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dooley J, Erickson M, Farr AG. Alterations of the medullary epithelial compartment in the Aire-deficient thymus: implications for programs of thymic epithelial differentiation. J Immunol. 2008;181:5225–5232. doi: 10.4049/jimmunol.181.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, Ikawa T, Satoh R, Kawamoto H, Mouri Y, Matsumoto M. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. The Journal of experimental medicine. 2008 doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]