Figure 1.
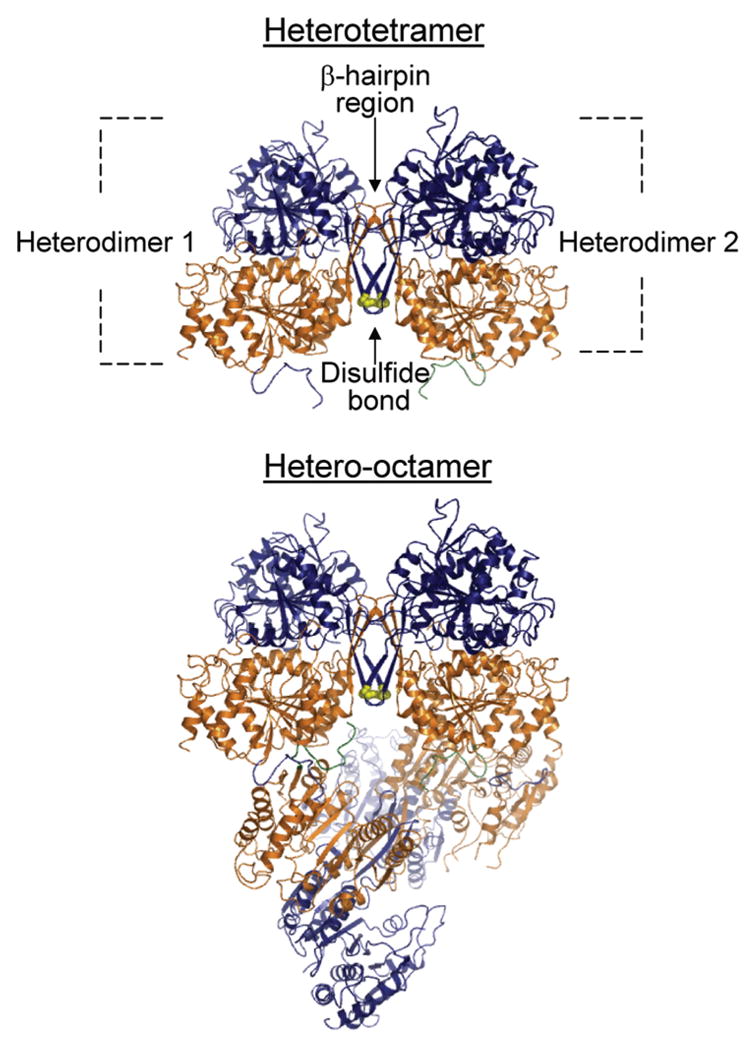
Organization of IDH1 and IDH2 subunits in the octameric holoenzyme. Shown is a depiction of the ligand-free structure determined for yeast IDH (15). Two heterodimers of IDH1 (orange) and IDH2 (navy) subunits form a heterotetramer primarily through interactions within a β-hairpin region, and two heterotetramers form the octamer primarily through contacts between IDH1 subunits in adjacent tetramers. The heterotetramers are offset from pseudo-222 symmetry by ~22°. IDH1 subunits form the internal core of the octamer with IDH2 subunits on the exterior. Cys-150 residues from IDH2 subunits in adjacent heterodimers are shown in yellow. Differences in environments around the Cys-150 residues in ligand-free and ligand-bound structures are presented and discussed in detail in ref 15.
