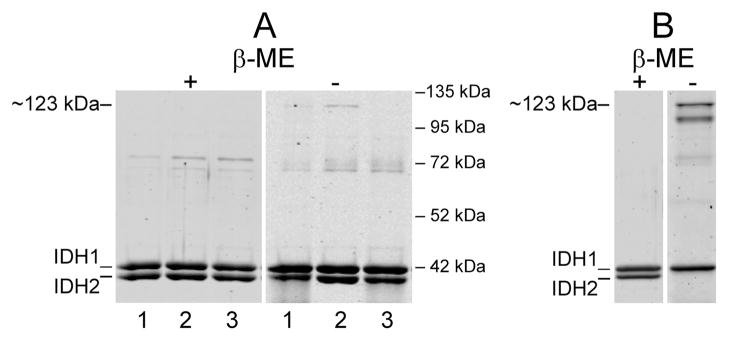
Figure 2.
Affinity-purified enzymes. (A) Samples (1.6 μg ea) of affinity-purified wild-type enzyme (lanes 1) and C56S/C242S and C150S mutant enzymes (lanes 2 and 3, respectively) were electrophoresed under reducing (plus β-mercaptoethanol = β-ME) or nonreducing (minus β-ME) conditions. Positions of molecular size standards are indicated on the right of the figure. (B) Samples (0.5 μg ea) of native wild-type IDH previously purified for crystallography experiments (15) and stored for ~6 months at −20 °C were electrophoresed under reducing or nonreducing conditions as indicated. The specific activity of the enzyme preparation was ~50% lower than that measured immediately after purification. The gels were stained with SYPRO Ruby.