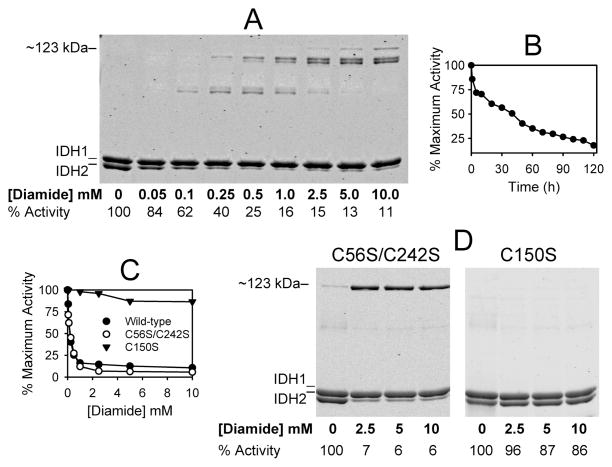
Figure 4.
Effects of diamide on electrophoretic properties of wild-type and mutant forms of IDH. (A) Samples (1 μM in 50 mM Tris-HCl at pH 7.4) of affinity-purified wild-type IDH were incubated with the indicated concentrations of diamide for 2 h on ice prior to nonreducing electrophoresis of 1.6 μg samples. IDH specific activity, expressed as the percent of the maximum activity measured, is indicated below each lane. (B) Decrease with time in specific activity (expressed as percent of the maximum activity measured) for wild-type IDH incubated with 0.5 mM diamide. (C) IDH specific activities were determined for wild-type (●), C56S/C242S (○), and C150S (▼;) enzymes following 2 h incubations with the indicated concentrations of diamide. Activities are expressed as the percent of the maximum activity measured. (D) Samples (1 μM in 50 mM Tris-HCl at pH 7.4) of affinity-purified C56S/C242S and C150S enzymes were incubated with the indicated concentrations of diamide for 2 h on ice prior to nonreducing electrophoresis of 1.6 μg samples. The gels were stained with SYPRO Ruby.