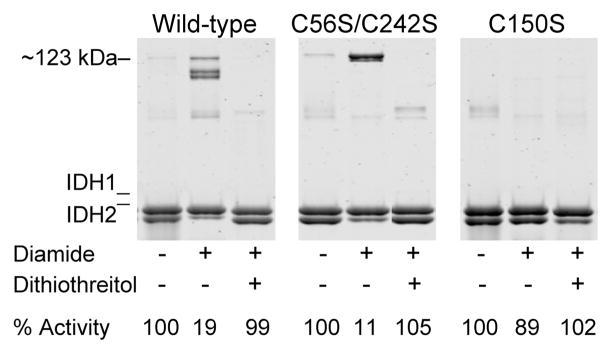
Figure 5.
Effects of dithiothreitol on disulfide bond formation in IDH. Samples (1 μM in 50 mM Tris-HCl at pH 7.4) of affinity-purified wild-type, C56S/C242S, and C150S enzymes as indicated were untreated (left lane in each panel), were incubated on ice with 5 mM diamide for 2 h (central lane in each panel), or were similarly incubated with diamide prior to the addition of 50 mM dithiothreitol and further incubation on ice for 30 min (right lane in each panel). Nonreducing electrophoresis was conducted using samples containing 1.6μg enzyme, and the gels were stained with SYPRO Ruby. IDH specific activity, expressed as the percent of that measured for the untreated enzyme, is indicated below each lane.