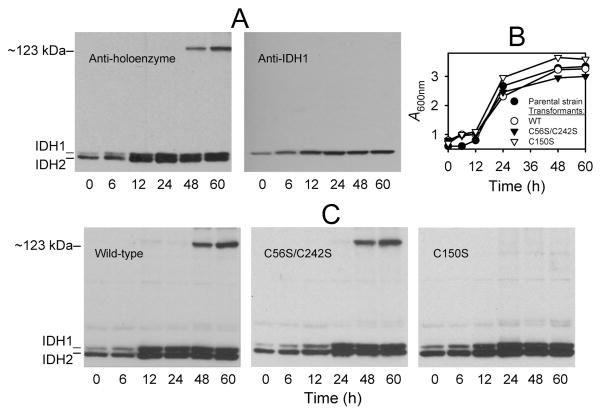
Figure 7.
Formation of a disulfide bond in IDH in stationary phase cells grown with acetate as the carbon source. (A) The parental yeast strain was cultivated using YP acetate medium, and protein extracts (~5μg ea) from cells harvested by trichloroacetic acid precipitation at the indicated times were electrophoresed on two gels under nonreducing conditions. One gel (left panel) was used for immunoblot analysis with an antiserum for the IDH holoenzyme (3), and the other gel (right panel) was used for immunoblot analysis with an antiserum specific for the IDH1 subunit (30). (B) Growth of cultures of the parental yeast strain (●) and of transformants expressing the wild-type (○), C56S/C242S (▼;), or C150S (∇) enzymes in YP acetate medium was monitored as A600 nm. (C) Protein samples (~5μg ea) from YP acetate cultures of transformants expressing the wild-type or mutant IDH enzymes as indicated were electrophoresed under nonreducing conditions, and the gels were used for immunoblot analysis with an antiserum for the IDH holoenzyme.