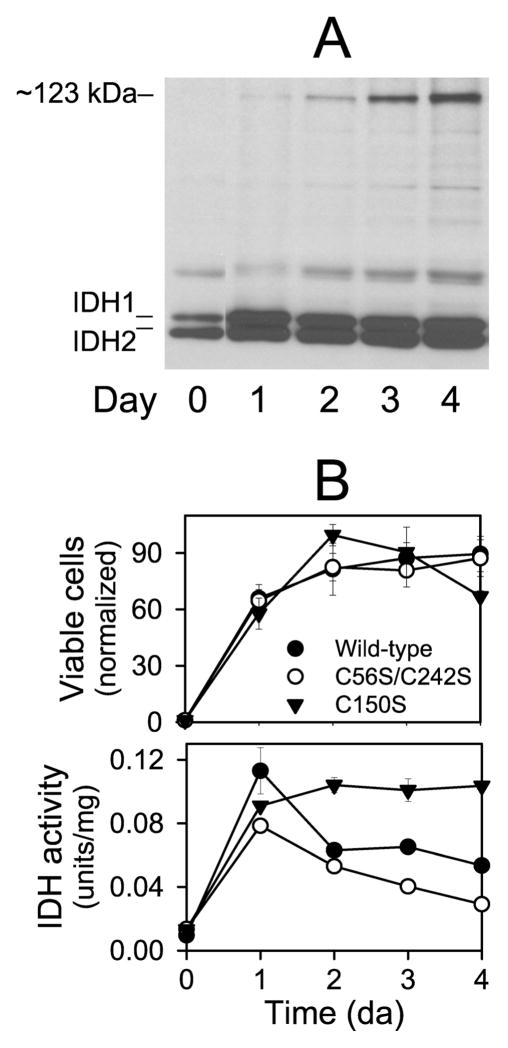
Figure 8.
Formation of a disulfide bond in IDH in stationary phase cells grown with ethanol as the carbon source. (A) Protein samples (~5μg ea) taken at indicated times during growth in YP ethanol medium of the yeast strain expressing wild-type IDH were electrophoresed under nonreducing conditions. The gel was used for immunoblot analysis with an antiserum for the IDH holoenzyme. (B) Samples of cultures of yeast strains expressing wild-type (●), C56S/C242S (○), or C150S (▼;) enzymes grown in YP ethanol medium were taken at indicated times to assess cellular viability (by plating dilutions on YP glucose plates and counting colonies after 3–5 days of growth at 30 °C) (top panel) or to determine cellular levels of IDH activity as described under Experimental Procedures (bottom panel). Cell numbers are normalized relative to numbers at time 0 (set at 1). IDH activity is expressed relative to mg cellular protein.