Abstract
Groups of male B6C3F1 mice were exposed by inhalation to 0, 25, 50, 100 or 200 p.p.m. ethylene oxide (EO) for up to 48 weeks (6 hours/day, 5 days/week). Animals were sacrificed at 6, 12, 24 and 48 weeks after the start of the exposure for analyses of reciprocal translocations in peripheral blood lymphocytes and germ cells. The frequency of the total chromosomal aberrations in the peripheral blood lymphocytes was significantly increased at the 100 and 200 p.p.m. exposure concentrations at the 12-week time point, at 50, 100 and 200 p.p.m. at the 24-week time point and at all EO concentrations at the 48-week time point. The frequency of stable reciprocal translocations, which can be used as biomarkers, was increased (P < 0.05) at 100 and 200 p.p.m. at the 12-week time point, at 100 and 200 p.p.m. at the 24-week time point and at 50, 100 and 200 p.p.m. at the 48-week time point. No statistically significant increase could be observed in translocation frequencies at the 6-week time point in the peripheral blood lymphocytes. The exposure–response curves were non-linear when the frequencies of translocations were plotted against EO exposure durations or against EO exposure concentrations. There was no effect of exposure concentration rate on reciprocal translocation frequency. Reciprocal translocations induced in spermatogonial stem cells (observed at the sprematocyte stage) showed significant increases in translocation frequencies over controls at all EO concentrations at 48 weeks. However, increases were small and they did not occur in a dose-responsive manner. The statistically significant increase observed at 12 weeks in the spermatocytes was equivocal. This study provides low-level chronic exposure somatic cytogenetic data generated in mice that can be used to support the shape of the tumour dose–response in rodents and humans The germ cell cytogenetic data are discussed in terms of its relevance for a threshold response for genetic effects at low exposures.
Introduction
Ethylene oxide (EO) (CAS No. 75-21-8) is a high volume production chemical that is used as an industrial intermediate and a sterilant for medical equipment. It is regulated by the US Environmental Protection Agency as a hazardous air pollutant under the Clean Air Act (1). It has been concluded to be carcinogenic to rodents and humans and classified as a Group 1 chemical (carcinogenic to humans) by the International Agency for Research on Cancer (IARC) (2). This classification was based on evidence, including limited data in humans for carcinogenicity, sufficient evidence in experimental animals and data for surrogate biomarkers of cancer (so-called mechanistic data). These surrogate biomarkers of cancer included formation of DNA adducts and genotoxicity in EO-exposed humans and rodents. In biomonitoring studies, it has been reported that cohorts of EO-exposed workers showed increased frequencies of several chromosomal aberration endpoints relative to unexposed workers (3). The mortality from lymphatic and haematopoietic cancer was also marginally elevated (2). IARC recently reassessed the carcinogenicity of EO, reconfirming the Group 1 classification (4, 5). The National Toxicology Program (NTP) has classified EO as ‘known to be a human carcinogen’ based on sufficient evidence of carcinogenicity from studies in humans, which included a combination of epidemiological and mechanistic investigations indicating a causal relationship between exposure to EO and human cancer (6). EO has also been subjected to several attempts at genetic risk assessment (7–9). However, these initial attempts suffered from several data gaps (10, 11), and it has been recommended that genetic risk assessments for genotoxic chemicals, such as EO, should be based on chronic exposures covering the entire cycle of spermatogenesis of the test organism (11).
EO is a known alkylating chemical agent and direct-acting mutagen, which reacts with nucleophilic molecules to produce DNA adducts, gene mutations and clastogenic damage. Its mutagenicity in somatic and germ cells has been clearly and repeatedly demonstrated in a variety of test systems, including mouse and rat assays. It has also been reported to have mutagenic effects in humans (2, 3, 7, 12, 13). Heritable reciprocal translocations are persistent stable chromosome alterations and are therefore of high importance for generating weight-of-evidence data for describing a potential role for mutagenicity in cancer risk assessments
In a study by Generoso et al. (8), male mice exposed to EO by inhalation at a range of 165–300 p.p.m. for a total of 8.5 weeks were found to have a significant increase in dominant lethal mutations at exposure levels of ≥204 p.p.m. and in the frequencies of reciprocal translocations in germ cells at all exposure levels. However, the incidence of heritable translocations did not show a linear dose–response relationship over the concentration range administered. Since the response was non-linear, and the lowest exposure concentration was 165 p.p.m., it was not possible to extrapolate the dose–response curve for reliably estimating induced effects at exposure levels approximating a few p.p.m. or, in reality, any exposure level <165 p.p.m. Furthermore, this study assessed post-meiotic germ cells as the target cells because these had been shown previously to be the most sensitive for the relatively large acute EO concentrations that were administered.
The purpose of the present study was to chronically expose male B6C3F1 mice to EO by inhalation and thereby provide data that could be used for genetic and cancer risk assessments through a parallelogram approach. We considered chronic low-level exposures to be more relevant for human risk assessment than acute high-level exposures. Since low chronic exposures present a hazard for male spermatogonial stem cells, which are present throughout the reproductive lifetime of the individual, these cells constitute the appropriate target cell population for assessment of heritable stable chromosome damage (11, 14). These requirements were achieved by conducting chronic (up to 48 weeks) relatively low-level (25–200 p.p.m.) inhalation exposures (compared to most published studies of somatic and germ cell mutagenicity, although the occupational exposure limit is 1 p.p.m. and considerably lower than the lowest concentration used in the present study). The genetic endpoint chosen was potentially heritable reciprocal translocations observed in spermatocytes and in peripheral blood lymphocytes. This approach was chosen as a necessary component of a genetic risk assessment model since somatic cell data (for peripheral blood lymphocytes) are largely what will be available for humans. It was also pertinent to use data on reciprocal translocations as being informative to dose–response for cancer risk assessment. We believe that the data generated in this study are more representative of human exposure than any of the previously published rodent cytogenetic studies. To our knowledge, this was also the first attempt to use a chronic chemical exposure to simultaneously assess genetic alterations in the germ and somatic cell populations in the same animal.
Materials and Methods
EO test compound
EO (CAS No. 75-21-8), chemical purity 99%, was obtained as a liquid in cylinders from ARC Chemical Division, Balchem Corp., State Hill, NY, USA. EO was considered to be stable at normal room temperature for the duration of the study based on the manufacturer's stability information.
Animals
Male B6C3F1 mice were purchased from Charles River Laboratories, Raleigh, NC, USA. The mice were 6 weeks ± 3 days of age upon arrival. The animals were acclimated for 10–14 days, randomized by weight using the PATH/TOX data acquisition and study management system software (Xybion Electronic Systems, San Diego, CA, USA) and divided into exposure and air-control groups for each of the four time points examined. The study facility was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International, and all procedures involving live animals were approved by the Institutional Animal Care and Use Committee (IACUC) and consistent with the Guide for the Care and Use of Laboratory Animals (15). The study-specific protocol was approved by the IACUC.
EO exposure levels
EO exposure levels of 25, 50, 100 or 200 p.p.m. were chosen based on considerations of the NTP carcinogenicity bioassay using B6C3F1 mice exposed to 50 or 100 p.p.m. EO for 2 years (16), the frequency of heritable translocations at levels of EO ≥167 p.p.m. (8) and EO-induced DNA adduct levels (N7-hydroxyethyl-guanine) in mice exposed to ≤100 p.p.m. EO (17).
Inhalation exposures to EO
Four groups of 44 male mice, ∼8 weeks old at the initiation of the exposure, were given whole-body exposures of 25, 50, 100 or 200 p.p.m. EO for 6, 12, 24 or 48 weeks (6 hours/day, 5 days/week). The air-control mice were exposed to clean air in a separate chamber in the same suite of rooms. EO was used as a model carcinogen and mutagen and so no positive control was required. The animals in each exposure group and in the air-control group were housed individually in wire mesh stainless steel cages inside a 1-m3 inhalation chamber (H1000; Lab Products, Seaford, DE, USA). The mice were given access to water ad libitum but food was removed during the 6-hour exposure period. Between each 6-hour exposure period and during weekends, the mice were fed NIH-07 certified feed and water ad libitum.
EO concentrations in the exposure chambers were monitored using infrared spectrophotometers (MIRAN 1A; The Foxboro Co., Foxboro, MA, USA). During the initial phase of the study, pressure and relative humidity were interfering with the air-control chamber concentration monitoring (baseline drift) and so a gas chromatograph (Model 5890 Series II; Hewlett–Packard Co., Palo Alto, CA, USA) equipped with a flame ionization detector was used to concurrently monitor the air-control chamber. Concentrations were measured at least once per hour from each of the five chambers.
Necropsy and tissue collection
Seven of the 11 animals from each exposure group (0, 25, 50, 100 or 200 p.p.m. EO) were sacrificed 3 days or earlier after the final exposure day for the 6-, 12-, 24- and 48-week time points. The mice were anesthetized with phenobarbital for collection of cardiac blood prior to asphyxiation using carbon dioxide followed by exsanguination and necropsy. The remaining four mice per dose group were allocated for other purposes and data collected from them are not included in this article.
Blood collection, lymphocyte culturing and preparation of metaphase cells
Lymphocyte cultures were prepared according to the method of Erexson and Kligerman (18), with some minor modifications. Cardiac blood (1–1.5 ml) was collected from anesthetized animals in heparinized syringes and mixed with an equal volume of sterile phosphate-buffered saline (Sigma-Aldrich, St Louis, MO, USA), and the leucocyte layer was immediately separated by centrifugation for 30 min at 400g using lymphocyte separation medium (Organon Teknika Corporation, Durham, NC, USA) according to the manufacturer's instructions. The isolated leucocyte layer was washed three times with 2% heat-inactivated foetal bovine serum, and the cells were used to set up 1-mm cultures of ∼5 × 105 cells each in complete RPMI 1640 (with L-glutamine and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) medium consisting of 20% foetal calf serum, an additional 2 mM L-glutamine and 100 U penicillin–streptomycin (all components from GIBCO BRL, Grand Island, NY, USA). Approximately, 5 μg/ml phytohaemagglutinin (Murex Diagnostic Ltd, Dartford, UK) was used as the mitogen. Two 1 ml lymphocyte cultures per animal were initiated and incubated undisturbed in loosely capped 6-ml sterile polystyrene tubes for 48 hours at 37°C in a humidified 5% CO2 atmosphere. Four hours prior to harvest, Karyomax Colcemid® (0.5 μg/ml, GIBCO BRL) was added to arrest cell division at metaphase. The cells were collected by centrifugation, treated for 3 min at 37°C with a 1:2 sodium citrate (0.03 M): potassium chloride (0.075 M) hypotonic solution and fixed three times with 1:3 glacial acetic acid: methanol fixative (all reagents from Sigma-Aldrich). Cells were stored refrigerated until slides were made. In order to optimize metaphase quality and chromosome spreading for the fluorescence in situ hybridization (FISH) procedure, the cell preparations were placed on super-clean microscope slides (Superfrost, Fisher Scientific, Pittsburgh, PA, USA) and the final fixative was evaporated from the slides aided by warm steam to provide high-quality metaphase spreads that would ensure optimal hybridization with the painting probes.
Fluorescence in situ hybridization
Whole-chromosome FISH was used for the detection and quantification of reciprocal translocations in lymphocytes. A cocktail of commercially available chromosome-specific probes for Chromosomes 1 and 3 (labelled with digoxigenin) and 2 and 8 (labelled with biotin) were used for the hybridization according to the manufacturer's instructions (Oncor, Gaithersburg, MD, USA). These four chromosome pairs represent ∼25% of the male mouse genome, The metaphase chromosomes on the prepared slides (aged for some days) were denatured in coplin jars containing a solution of 300 mM sodium chloride, 30 mM sodium citrate, pH 5.3 (2× SSC) for 2 min at 72°C and dehydrated in cold 70, 80 and 100% ethanol for 2 min each. The slides were air-dried at room temperature prior to hybridization. The hybridization mixture for each slide consisted of the premixed chromosome-specific probe in a 50% formamide/2× SSC hybridization buffer. The probe was denatured at 72°C for 10 min and preannealed at 37°C for 2 hours. The hybridization mixture was then applied to the metaphase slides. The slides were covered with glass coverslips, placed flat in a sealed plastic box with moisturized paper towels to ensure 100% humidity and kept at 37°C overnight. After hybridization, the coverslips were removed and the slides washed in 2× SSC for 5 min at 72°C and in 1× phosphate-buffered detergent (PBD; Oncor) solution for a few minutes at room temperature. Sixty microlitres of a Fluorescein isothiocyanate (FITC)–avidin/rhodamine anti-digoxigenin detection solution was then added to each still slightly moist slide, coverslipped and incubated at room temperature for 5 min. The slides were washed three times in PBD at room temperature for 2 min each and counterstained with 10 μl 4′,6′-diamino-2-phenylindole (DAPI) per slide before placing coverslips on the slides.
Meiotic spermatocyte preparations
Isolation of germ cells for meiotic spermatocyte preparations was according to Brewen and Preston (19) as modified by Wessels-Kaalen (20). One testis was collected in 2.2% sodium citrate (Sigma-Aldrich) and the seminiferous tubules were released through an incision in the tunica albuginea. The tubules were macerated on a glass surface by rolling with a silicone rubber-covered steel bar. The macerated material was placed in a tube with ∼5 ml 2.2% sodium citrate to allow the larger pieces of material to settle. The supernatant was removed and the tubular cells were collected by centrifugation, resuspended in 1 ml hypotonic solution (1% sodium citrate) and incubated for 15 min at room temperature. The cells were collected by centrifugation, the pellet was resuspended in the 0.5 ml remaining supernatant, and the resulting cell suspension was fixed three times with 1:3 glacial acetic acid: methanol fixative (both from Sigma-Aldrich). The samples were stored refrigerated until placed on slides and stained with 4% Giemsa (Sigma-Aldrich). The slides were then covered by coverslips and permanently sealed.
Scoring of metaphase cells in lymphocytes and of multivalents in germ cells
FISH-painted metaphases in peripheral blood lymphocytes were analysed with a Nikon Microphot-FXA fluorescence microscope using a triple-bandpass filter set for FITC/Texas Red/DAPI for visualization of the painted chromosomes. DAPI was used as a fluorescing DNA counterstain. Well-spread metaphases that appeared to have completely painted chromosomes with observable centromeres were scored for reciprocal translocations involving painted and unpainted chromosomes. Other aberrations analysed that involved painted chromosomes were terminal translocations, insertions, and dicentrics; these are all exchange-type aberrations that would have been induced in vivo. Chromosome aberrations that did not involve painted chromosomes were excluded from the analyses. Meiotic reciprocal translocations, appearing as multivalents at the diplotene/diakinesis stage of spermatogenesis, were analysed in Giemsa-stained cells (21). All cells meeting the criteria for scoring were included in the cytogenetic analyses, and all slides were analysed under code.
It is important to note that given the progressive nature of spermatogenesis, reciprocal translocations induced by long-term chronic treatment will be induced almost exclusively in spermatogonial stem cells although they are analysed at the diplotene/diakinesis stage of spermatocyte meiosis, the optimal stage for identification of reciprocal translocations.
Statistical analyses
Statistical calculations of means and standard errors were weighted to adjust for different numbers of lymphocyte metaphases or germ cell spermatocytes counted per animal. The statistical analyses to compare the results from the air-control and EO exposure groups were conducted by Fisher's exact test (two tailed) and the Cochran–Armitage trend test. All significance was judged at a P value of 0.05.
Results
EO exposure levels and conditions
The target exposure levels for each EO exposure chamber and for air-controls were 0, 25, 50, 100 and 200 p.p.m. 6 hours/day, 5 days/week for up to 48 weeks. The overall average measured EO concentrations (in p.p.m. ± standard deviation) in each exposure chamber for the entire 48-week exposure period were 0 ± 0, 25 ± 2, 50 ± 2, 100 ± 3 and 199 ± 4 p.p.m. for the target concentrations, respectively. Daily average temperature and relative humidity for chambers monitored for the entire 48-week exposure period were between 22.2 and 22.4°C and 43 and 44%, respectively. The air-control mice were exposed to clean air of the same temperature, relative humidity and airflow as delivered to the EO-exposed animals.
Reciprocal translocations and total aberrations in peripheral blood lymphocytes
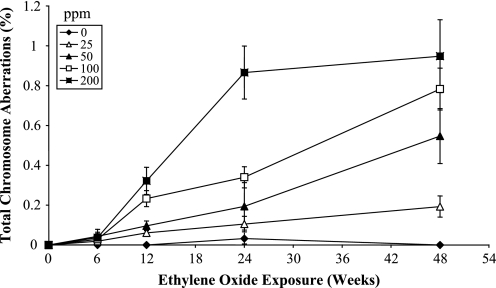
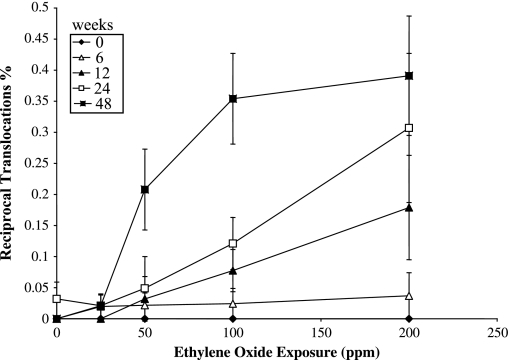
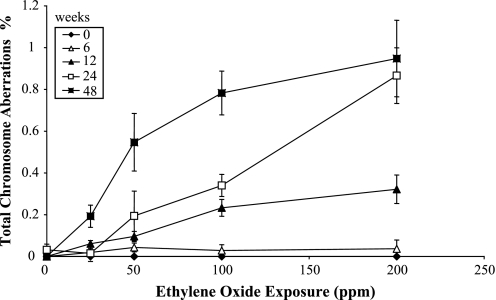
Data for reciprocal translocations in the peripheral blood lymphocytes are shown in Table I, together with data for the other types of aberrations analysed. The frequency of reciprocal translocations was significantly (P < 0.05) increased in relation to the air-control at the 12-week time point at the 100 and 200 p.p.m. exposure concentrations, at the 24-week time point at the 100 and 200 p.p.m. exposure concentrations and at 50, 100 and 200 p.p.m. exposure concentrations at the final 48-week time point. The same pattern of induced chromosome aberrations was seen when all observed aberration types were included, with the exception of a stronger response in terms of frequency of aberrations, with even a significant response at the lowest exposure concentration of 25 p.p.m. at the 48-week time point. The reciprocal translocation data are shown as a dose–response curve in Figure 1, and the data for total aberrations in Figure 2, where total dose is represented by exposure duration for each delivered EO concentration. In addition, the reciprocal translocation and total aberration data are plotted as a function of exposure concentration (p.p.m.), for each exposure duration, in Figures 3 and 4, respectively. At the 6-week exposure duration and the 25 p.p.m. exposure concentration, there were only small increases in translocation frequency. The dose–response curves at the higher concentrations and durations were non-linear. There was no difference in the frequency of reciprocal translocations when the same total dose was delivered at different concentrations (e.g. the frequency was essentially the same if 25 p.p.m. was given for 48 weeks or 100 p.p.m. for 12 weeks).
Table I.
The frequency of reciprocal translocations and other aberration types following EO exposure
| Exposure (weeks) | Concentration (p.p.m.) | Animals (n) | Chromosomal aberrations |
Metaphases scored (n) | Reciprocal translocations (%) (mean ± SE) | Total aberrations (%) (mean ± SE) | |||
| RT | TT | INS | DIC | ||||||
| 6 | 0 | 6 | 0 | 0 | 0 | 0 | 3243 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| 25 | 7 | 1 | 0 | 0 | 0 | 5214 | 0.019 ± 0.019 | 0.019 ± 0.019 | |
| 50 | 7 | 1 | 0 | 1 | 0 | 4619 | 0.021 ± 0.020 | 0.043 ± 0.021 | |
| 100 | 5 | 1 | 0 | 0 | 0 | 3529 | 0.028 ± 0.024 | 0.028 ± 0.028 | |
| 200 | 4 | 1 | 0 | 0 | 0 | 2701 | 0.037 ± 0.037 | 0.037 ± 0.042 | |
| 12 | 0 | 4 | 0 | 0 | 0 | 0 | 2976 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| 25 | 5 | 0 | 2 | 0 | 0 | 3285 | 0.000 ± 0.000 | 0.061 ± 0.016 | |
| 50 | 5 | 1 | 1 | 1 | 0 | 3130 | 0.032 ± 0.036 | 0.070 ± 0.024 | |
| 100 | 4 | 2 | 0 | 2 | 2 | 2508 | 0.080 ± 0.034* | 0.239 ± 0.040* | |
| 200 | 4 | 5 | 0 | 2 | 2 | 2795 | 0.179 ± 0.084* | 0.322 ± 0.068* | |
| 24 | 0 | 4 | 1 | 0 | 0 | 0 | 3134 | 0.032 ± 0.027 | 0.032 ± 0.027 |
| 25 | 7 | 2 | 1 | 1 | 2 | 4770 | 0.042 ± 0.019 | 0.126 ± 0.039 | |
| 50 | 7 | 2 | 3 | 2 | 3 | 4117 | 0.049 ± 0.051 | 0.243 ± 0.119* | |
| 100 | 6 | 5 | 3 | 2 | 4 | 4120 | 0.121 ± 0.042* | 0.340 ± 0.053* | |
| 200 | 5 | 11 | 2 | 4 | 13 | 3581 | 0.307 ± 0.120* | 0.838 ± 0.133* | |
| 48 | 0 | 7 | 0 | 0 | 0 | 0 | 4066 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| 25 | 8 | 1 | 1 | 3 | 3 | 4672 | 0.021 ± 0.019 | 0.171 ± 0.053*# | |
| 50 | 7 | 11 | 5 | 5 | 8 | 5300 | 0.204 ± 0.065* | 0.547 ± 0.138*# | |
| 100 | 7 | 14 | 6 | 0 | 8 | 3883 | 0.361 ± 0.073* | 0.721 ± 0.105*# | |
| 200 | 6 | 19 | 5 | 7 | 16 | 4854 | 0.391 ± 0.096* | 0.968 ± 0.183*# | |
RT, reciprocal translocations; TT, terminal translocations; INS, insertions; DIC, dicentrics.
*P < 0.05 (Fisher's exact test), #P < 0.05 (Cochran–Armitage test).
Fig. 1.
Frequencies of reciprocal translocations (%) in mouse peripheral lymphocytes as a function of exposure time (weeks) and EO exposure concentration (p.p.m.).
Fig. 2.
Frequencies of total chromosome aberrations (%) involving painted chromosomes in mouse peripheral lymphocytes as a function of exposure time (weeks) and EO exposure concentration (p.p.m.).
Fig. 3.
Frequencies of reciprocal translocations (%) in mouse peripheral lymphocytes as a function of EO exposure concentration (p.p.m.) and exposure time (weeks).
Fig. 4.
Frequencies of total chromosome aberrations (%) involving painted chromosomes in mouse peripheral lymphocytes as a function of EO exposure concentration (p.p.m.) and exposure time (weeks).
Reciprocal translocations observed in spermatocytes
Data for reciprocal translocations observed in the primary spermatocytes are shown in Table II. As mentioned above, these translocations are almost exclusively induced in spermatogonia and specifically in spermatogonial stem cells at the longer exposures (12 weeks and longer). The frequency of reciprocal translocations was (P < 0.05) increased at the 48-week time point for all concentration levels, without any clear dose–response. A statistically significant trend was seen at the 100 p.p.m. exposure level at the 12-week time point, but the response is considered equivocal as discussed below.
Table II.
The frequency of reciprocal translocations observed in mouse spermatocytes following EO exposure
| Exposure (weeks) | Concentration (p.p.m.) | Animals (n) | Reciprocal translocations (n) | Metaphases scored (n) | Reciprocal translocations (%) (mean ± SE) |
| 6 | 0 | 6 | 0 | 775 | 0.000 ± 0.000 |
| 25 | 7 | 0 | 668 | 0.000 ± 0.000 | |
| 50 | 7 | 1 | 670 | 0.150 ± 0.124 | |
| 100 | 6 | 0 | 810 | 0.000 ± 0.000 | |
| 200 | 3 | 0 | 334 | 0.000 ± 0.000 | |
| 12 | 0 | 5 | 0 | 653 | 0.000 ± 0.000 |
| 25 | 5 | 1 | 340 | 0.294 ± 0.252 | |
| 50 | 5 | 0 | 388 | 0.000 ± 0.000 | |
| 100 | 5 | 1 | 401 | 0.249 ± 0.245 | |
| 200 | 5 | 3 | 281 | 1.068 ± 0.302# | |
| 24 | 0 | 4 | 0 | 291 | 0.000 ± 0.000 |
| 25 | 7 | 3 | 423 | 0.709 ± 0.245 | |
| 50 | 6 | 2 | 362 | 0.553 ± 0.335 | |
| 100 | 7 | 3 | 393 | 0.763 ± 0.288* | |
| 200 | 4 | 2 | 284 | 0.704 ± 0.382 | |
| 48 | 0 | 8 | 0 | 427 | 0.000 ± 0.000 |
| 25 | 8 | 5 | 621 | 0.805 ± 0.248* | |
| 50 | 7 | 3 | 395 | 0.759 ± 0.278* | |
| 100 | 8 | 5 | 469 | 1.066 ± 0.298* | |
| 200 | 7 | 5 | 443 | 1.129 ± 0.308* |
*P < 0.05 (Fisher's exact test), #P < 0.05 (Cochran–Armitage test).
Discussion
Male mice only were used in our study because the aim was to provide data that could be used for comparison with previously published germ cell data for EO (22–25). The B6C3F1 mouse strain was chosen because it was the one that was used in the chronic bioassays for EO carcinogenicity (16).
The development of FISH methods (26), and particularly the development of mouse painting probes, allows selective visualization of individual chromosome pairs (27) that provides an objective and accurate means for the detection of chromosomal translocations and mutations. For this application, aberrations are considered to be randomly distributed across the genome, so any painting probe can be used for detection, although the number of mutational events is proportional to the total length of the chromosome probes used. We increased the detection power by using a cocktail of digoxigenin- and biotin-labelled commercially available whole chromosome painting probes for Chromosomes 1, 2, 3 and 8. Using these four chromosomes as indicators, we were able to monitor the cumulative dose–response caused by increasing exposure concentration over time as indicators of clastogenic damage. This set of four chromosome pairs represents ∼25% of the male mouse genome.
Inhalation exposure to EO for up to 48 weeks induced reciprocal translocations and other observable aberrations in the peripheral blood lymphocytes with increases being proportional to exposure duration. This indicates the suitability of this cell type as a target for biological monitoring. The data also show a detectable increase in the frequency of potentially heritable translocations in spermatogonial stem cells but only at the 48-week time point. This response is in contrast to the lymphocyte response in the same animals and, although rather unlikely for EO, could be the result of the absorption of EO being reduced by the blood–testis barrier thereby protecting the cells from induced DNA damage. This would result in a considerably lower dose to the testis than to the peripheral blood, as also suggested by dosimetry data for EO (17) and propylene oxide (28). Alternatively, and perhaps more likely, the great majority of the EO-induced DNA damage could be repaired prior to meiotic DNA synthesis or that cells containing chromosome damage induced in early spermatogonial cell stages are removed from the cycling population. DNA synthesis is required to convert EO-induced DNA damage into chromosomal aberrations (3). In this regard, it is important to note that the great majority of the chronic exposure will have been to spermatogonial stem cells, given the duration of the exposures (6 weeks and longer) and the duration of spermatogenesis in mice. The S-phase in mouse spematogonial stem cells lasts a few hours even though the duration of the total cell cycle is ∼9 days, with the great majority being G1 (29). The significance of this is that there is a considerable amount of time for repair of DNA damage in G1 before the cell enters the S-phase, except for those very few cells that are close to the start of S or that are already in S (estimated to be a fraction of a percent of the total). Thus, the frequency of reciprocal translocations induced by EO in spermatogonial stem cells is predicted to be rather low, as indeed is the case in our study. This is in contrast to what is observed for radiation exposures for which significant increases in reciprocal translocations were observed in spermatocytes derived from irradiated stem cells. The reason for this is that radiation-induced chromosome aberrations can be induced in the absence of DNA synthesis—namely, at any stage of the cell cycle (30).
In the present study, the shapes of the dose–response curves for reciprocal translocations and total aberrations involving painted chromosomes (i.e. including terminal translocations insertions and dicentrics that are all exchange aberrations induced in vivo) in peripheral blood lymphocytes are identical. This demonstrates that the combined response to reciprocal translocations and the other recorded exchange aberrations is equal to the addition of the frequencies of the separate classes. The overall shape of dose–response curves for each delivered concentration was non-linear, with a clear saturation for the 200 p.p.m. concentration. There is no apparent explanation for the saturation of response at this time, given that the translocation frequencies are still relatively low at the 200 p.p.m. exposure level. Exposure for 6 or 12 weeks leads to only a marginal and not statistically significant induction of reciprocal translocations, with a statistically significant (P < 0.05) trend at the 12-week time point. Clear statistically significant increases were seen at the 24- and 48-week time points. The dose–response curve at the 24-week time point shows no significant increase over the air-control at exposure concentrations of 25 and 50 p.p.m. with a linear response for the higher two concentrations. However, based on the ratio of the % reciprocal translocations to the exposure concentration, an index of the efficiency of reciprocal translocation formation increased from 0.34 × 10−3 to 1.38 × 10−3 as the exposure concentration was increased from 50 to 200 p.p.m. EO. Thus, based on this measure, the dose–response curve for reciprocal translocations was sublinear over the dose range of 50–200 p.p.m. at the 24-week time point. The curve for the 48-week time point is also clearly non-linear with no significant increase over the air-control at 25 p.p.m., a steep linear increase from >25 to 100 p.p.m. and a flattening of the curve between 100 and 200 p.p.m.
The heritable translocation data in the present study were generated in the same strain of mice (B6C3F1) as those used in a parallel study with Big Blue mice for assessing lacI mutation frequency in the bone marrow and testes (31). In the bone marrow, the lacI mutant frequency was significantly increased at the two highest exposure levels (100 and 200 p.p.m.) at the 48-week time point. In the testes, the lacI mutant frequency was increased at a single exposure level of 200 p.p.m. for 24 weeks. At 48 weeks, the overall lacI mutation frequency in testes was significantly increased to an equal degree at 25, 50 and 100 p.p.m. EO but not at 200 p.p.m. These results generally correspond to our findings of the induction of potentially heritable translocations in spermatogonial cells.
Data from other rodent studies show conflicting information regarding the ability of EO to induce cytogenetic effects. Intraperitoneal injection of EO induced dominant lethal mutations and reciprocal translocations in post-meiotic male germ cells (22), and inhalation exposure of post-meiotic cells to EO led to a non-linear exposure-related increase in dominant lethal mutations and heritable translocations at high exposure levels of >167 p.p.m. (8, 23). In contrast, two studies failed to show increased frequencies of EO-induced clastogenic damage in somatic cells of rats. No increase in chromosome aberrations was observed in peripheral blood lymphocytes in rats acutely exposed by inhalation to EO at a concentration range of 50–450 p.p.m. for 1- or 3-day exposure (6 hours/day) (32); there was a significant increase in sister chromatid exchanges (SCEs). Similarly, there was no increase in chromosome aberrations in rats exposed to 150 p.p.m. EO for the duration of 1, 2, 3 or 4 weeks (5 days/week, 6 hours/day) but there was an increase in SCE (33). Neither chromosome aberrations nor translocations analysed by FISH were observed in rats exposed to 50, 100 or 200 p.p.m. EO for 4 weeks (5 days/week, 6 hours/day) (34, 35). It is feasible that rats and mice differ quite significantly in their mutagenic responses to EO, and this difference could be related to differences in pharmacokinetics between the species (36).
In conclusion, we measured the translocation frequency in two target tissues of male B6C3F1 mice exposed to EO by inhalation for up to 48 weeks. Under the conditions of this study, heritable stable chromosomal changes were induced in the peripheral blood lymphocytes in a non-linear dose-responsive manner, with significant increases at 12, 24 and 48 weeks of exposure only at chronic exposure concentrations >50 p.p.m. Significant increases were observed at 50 p.p.m. for exposure times of 24 and 48 weeks. These data also demonstrate that most of the EO exposures that produced distinct responses in the somatic cells did not do so in spermatogonial stem cells of the testis in the very same animals. The frequencies of translocations induced in spermatogonial stem cells were significantly increased for all concentrations at 48 weeks, although these increases were very similar for all four concentrations. There were also significant increases for 100 p.p.m. at 24 weeks of exposure and for 200 p.p.m. at 12 weeks.
These data demonstrate a dose–rate correlation for EO-exposed male B6C3F1 mice for the induction of reciprocal translocations and the increases in the incidence of tumours that were observed at concentration levels of >50 p.p.m. at ≥60 weeks in the NTP 2-year chronic EO inhalation bioassay (16). Thus, the data generated in the present study are informative as regards the shape of the dose–response curve for the type of chromosomal alteration (reciprocal translocations) that is transmissible and of significance in the formation of tumours and for assessing the potential for EO to be a germ cell mutagen at low chronic exposures. The use of the present data in the context of cancer risk assessment is currently most appropriate in a qualitative fashion, namely providing valuable input on the shape of the rodent and, more importantly, human tumour dose–response. This extrapolation depends upon reciprocal translocations induced in peripheral lymphocytes being a surrogate for a key event in tumour formation. This is a reasonable conclusion as described in the paper by Kirman et al. (37) in which the authors utilize mechanistic data, including those for reciprocal translocations, for cancer potency estimations for EO. If human data for reciprocal translocations induced in peripheral lymphocytes were available over a range of exposure concentrations, including some closer to those employed in the present study, it might be possible to use the rodent data in a more quantitative manner for an EO cancer risk assessment. The germ cell data presented in the present manuscript confirm the conclusion developed by Preston et al. (11) that based on mechanistic considerations, the dose–response curve for reciprocal translocations induced in spermatogonial stem cells by chronic EO exposures will depict a threshold at low exposure concentrations. Of particular note is that these low experimental concentrations are still several fold higher than the allowable occupational exposure level for EO. Finally, the reciprocal translocation data in somatic cells could provide a valuable parameter set for a biologically based dose–response model for cancer risk assessment. Thus, this study provides a number of most valuable data sets for subsequent risk assessment applications.
Funding
National Institutes of Health National Research Service Award Grant (ES055693-02 to E.M.D.).
Acknowledgments
We thank The Hamner Institutes for Health Sciences’ inhalation facility staff, animal care facility staff and necropsy staff for expert technical assistance. [Note: When this study was conducted, The Hamner Institutes for Health Sciences was called the Chemical Industry Institute of Toxicology.] Dr David T. Mage provided helpful discussions and assistance with statistical calculations. We thank Drs James Allen and Andrew Kligerman for their helpful review of the manuscript. This manuscript has been reviewed according to the policy of the US Environmental Protection Agency although it does not necessarily represent Agency regulatory policy (for R.J.P.).
Conflict of interest statement: None declared.
References
- 1.US Environmental Protection Agency (US EPA) Preliminary draft list of categories and subcategories under Section 112 of the Clean Air Act. Fed. Reg. 1991;56:28548–28557. [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) Some Industrial Chemicals: Summary of Data Reported and Evaluation. IARC. 1994;60:73–159. [Google Scholar]
- 3.Preston RJ. Cytogenetic effects of ethylene oxide, with an emphasis on population monitoring. Crit. Rev. Toxicol. 1999;29:263–282. doi: 10.1080/10408449991349212. [DOI] [PubMed] [Google Scholar]
- 4.Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Bouvard V, Altier A, Cogliano V. Carcinogenicity of 1,3-butadiene, ethylene oxide, vinyl chloride, vinyl fluoride, and vinyl bromide. Lancet Oncol. 2007;8:679–680. doi: 10.1016/s1470-2045(07)70235-8. [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risk to humans. 1,3-Butadiene, ethylene oxide, and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide) IARC. 2008;97:185–309. [PMC free article] [PubMed] [Google Scholar]
- 6.National Toxicology Program (NTP) Ninth Report on Carcinogens. Research Triangle Park, NC: US Department of Health and Human Services, Public Health Service, National Toxicology Program; 2001. http://ehp.niehs.nih.gov/roc/toc9.html (accessed December 6, 2008) [Google Scholar]
- 7.Dellarco VL, Generoso WM, Sega GA, Fowle JR, Jacobson-Kram D. Review of the mutagenicity of ethylene oxide. Environ. Mol. Mutagen. 1990;16:85–103. doi: 10.1002/em.2850160207. [DOI] [PubMed] [Google Scholar]
- 8.Generoso WM, Cain KT, Cornett CV, Cachiero NLT, Hughes LA. Concentration-response curves for ethylene oxide-induced heritable translocations and dominant lethal mutations. Environ. Mol. Mutagen. 1990;16:126–131. doi: 10.1002/em.2850160209. [DOI] [PubMed] [Google Scholar]
- 9.Rhomberg L, Dellarco VL, Siegel-Scott C, Dearfield KL, Jacobson-Kram D. Quantitative estimation of the genetic risk associated with the induction of heritable translocations at low-dose exposure: ethylene oxide as an example. Environ. Mol. Mutagen. 1990;16:104–125. doi: 10.1002/em.2850160208. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan AT, Preston RJ, Dellarco V, Ehrenberg L, Generoso W, Lewis S, Tates AD. Ethylene oxide: evaluation of genotoxicity data and an exploratory assessment of genetic risk. Mutat. Res. 1995;330:55–70. doi: 10.1016/0027-5107(95)00036-i. [DOI] [PubMed] [Google Scholar]
- 11.Preston RJ, Fennell TR, Leber AP, Sielken RL, Swenberg JA. Reconsideration of the genetic risk assessment from ethylene oxide exposures. Environ. Mol. Mutagen. 1996;26:189–202. doi: 10.1002/em.2850260303. [DOI] [PubMed] [Google Scholar]
- 12.Their R, Bolt HM. Carcinogenicity and genotoxicity of ethylene oxide: new aspects and recent advances. Crit. Rev. Toxicol. 2000;30:595–608. doi: 10.1080/10408440008951121. [DOI] [PubMed] [Google Scholar]
- 13.Kolman A, Chovanec M, Osterman-Golkar S. Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: update review (1990-2001) Mutat. Res. 2002;512:173–194. doi: 10.1016/s1383-5742(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 14.Preston RJ. Future of germ cell cytogenetics. Environ. Mol. Mutagen. 1994;23(Suppl. 24):54–58. doi: 10.1002/em.2850230613. [DOI] [PubMed] [Google Scholar]
- 15.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy of Sciences. National Academy Press; 1996. [Google Scholar]
- 16.National Toxicology Program (NTP) Toxicology and Carcinogenesis Studies of Ethylene Oxide in B6C3F1 Mice. Washington, DC: NTP Technical Report, USDHHS, US Government Printing Office; 1988. p. 326. [PubMed] [Google Scholar]
- 17.Walker VE, Fennell TR, Upton PB, MacNeela JP, Swenberg JA. Molecular dosimetry of DNA and hemoglobin adducts in mice and rats exposed to ethylene oxide. Environ. Health Perspect. 1992;99:11–17. doi: 10.1289/ehp.939911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erexson GL, Kligerman AD. A modified mouse peripheral blood lymphocyte culture system for cytogenetic analysis. Environ. Mol. Mutagen. 1987;10:377–386. doi: 10.1002/em.2850100407. [DOI] [PubMed] [Google Scholar]
- 19.Brewen JG, Preston RJ. Radiation-induced chromosome aberrations in somatic and germ cells of the male marmoset. Primates Med. 1978a;10:199–204. [PubMed] [Google Scholar]
- 20.Wessels-Kaalen MCA. A new procedure for meiotic preparations for mammalian testes. Mammal. Chromosome Newslett. 1984;25:66–67. [Google Scholar]
- 21.Brewen JG, Preston RJ. Analysis of chromosome aberrations in mammalian germ cells. In: Hollaender A, de Serres FJ, editors. Chemical Mutagens. Vol. 5. New York: Plenum Publishing; 1978b. [Google Scholar]
- 22.Generoso WM, Cain KT, Krishna M, Sheu CW, Gryder RM. Heritable translocation and dominant-lethal mutation induction with ethylene oxide in mice. Mutat. Res. 1980;73:133–142. doi: 10.1016/0027-5107(80)90142-6. [DOI] [PubMed] [Google Scholar]
- 23.Generoso WM, Cain KT, Hughes LA, Sega GA, Braden PW, Gosslee DG, Shelby MD. Ethylene oxide dose and dose-rate effects in the mouse dominant-lethal test. Environ. Mutagen. 1986;8:1–7. doi: 10.1002/em.2860080102. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SE, Barnett LB, Felton C, Johnson FM, Skow LC, Cacheiro N, Shelby MD. Dominant visible and electrophoretically expressed mutations induced in male mice exposed to ethylene oxide by inhalation. Environ. Mutagen. 1986;8:867–872. doi: 10.1002/em.2860080609. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro LR, Rabello-Gay MN, Salvadori DMF, Pereira CAB. Cytogenetic effects of inhaled ethylene oxide in somatic and germ cells of mice. Arch. Toxicol. 1987;59:332–335. doi: 10.1007/BF00295085. [DOI] [PubMed] [Google Scholar]
- 26.Swiger RR, Tucker JD. Fluorescence in situ hybridization. A brief review. Environ. Mol. Mut. 1996;27:245–254. doi: 10.1002/(SICI)1098-2280(1996)27:4<245::AID-EM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Breneman JW, Ramsey MJ, Lee DA, Eveleth GG, Minkler JI, Tucker JD. The development of chromosome-specific composite DNA probes for the mouse and their application to chromosome painting. Chromosoma. 1993;102:591–598. doi: 10.1007/BF00352306. [DOI] [PubMed] [Google Scholar]
- 28.Rios-Blanco M, Faller TH, Nakamura J, Kessler W, Kreutzer PE, Ranasinghe A, Filser JG, Swenberg JA. Quantitation of DNA and hemoglobin adducts and apurinic/apyrimidinic sites in tissues of F344 rats exposed to propylene oxide by inhalation. Carcinogenesis. 2000;21:2011–2018. doi: 10.1093/carcin/21.11.2011. [DOI] [PubMed] [Google Scholar]
- 29.Oakberg EF. Duration of spermatogenesis in the mouse. Nature. 1957;180:1137–1138. doi: 10.1038/1801137a0. [DOI] [PubMed] [Google Scholar]
- 30.Brewen JG, Preston RJ, Luippold HE. Radiation-induced translocations in spermatogonia. III. Effect of long-term chronic exposure to gamma-rays. Mutat. Res. 1979;61:405–409. doi: 10.1016/0027-5107(79)90146-5. [DOI] [PubMed] [Google Scholar]
- 31.Recio L, Donner M, Abernethy D, Pluta L, Oteen A-M, Wong BA, James A, Preston RJ. In vivo mutagenicity and mutation spectrum in the bone marrow and testes of B6C3F1 lacI transgenic mice following inhalation exposure to ethylene oxide. Mutagenesis. 2004;19:215–222. doi: 10.1093/mutage/geh017. [DOI] [PubMed] [Google Scholar]
- 32.Kligerman AD, Erexson GL, Phelps ME, Wilmer JL. Sister chromatid exchange induction in peripheral blood lymphocytes of rats exposed to ethylene oxide by inhalation. Mutat. Res. 1983;120:37–44. doi: 10.1016/0165-7992(83)90071-4. [DOI] [PubMed] [Google Scholar]
- 33.Preston RJ, Abernethy DJ. Studies of the induction of chromosomal aberration and sister chromatid exchange in rats exposed to styrene by inhalation. IARC Sci. Publ. 1993;127:225–233. [PubMed] [Google Scholar]
- 34.van Sittert NJ, Boogaard PJ, Natarajan AT, Tates AD, Ehrenberg LG, Tornqvist MA. Formation of DNA adducts and induction of mutagenic effects in rats following 4 weeks inhalation exposure to ethylene oxide as a basis for cancer risk assessment. Mutat. Res. 2000;447:27–48. doi: 10.1016/s0027-5107(99)00208-0. [DOI] [PubMed] [Google Scholar]
- 35.Lorenti-Garcia C, Darroudi F, Tates AD, Natarajan AT. Induction and persistence of micronuclei, sister-chromatid exchanges and chromosomal aberrations in splenocytes and bone-marrow cells of rats exposed to ethylene oxide. Mutat. Res. 2001;492:59–67. doi: 10.1016/s1383-5718(01)00149-8. [DOI] [PubMed] [Google Scholar]
- 36.Fennell TR, Brown CD. A physiologically based pharmacokinetic model for ethylene oxide in mouse, rat, and human. Toxicol. Appl. Pharmacol. 2001;173:161–175. doi: 10.1006/taap.2001.9184. [DOI] [PubMed] [Google Scholar]
- 37.Kirman CR, Sweeney LH, Teta MJ, Sielken RL, Valdez-Flores C, Albertini RJ, Gargas ML. Addressing non-linearity in the exposure-response relationship for a genotoxic carcinogen: cancer potency estimates for ethylene oxide. Risk Anal. 2004;24:1165–1183. doi: 10.1111/j.0272-4332.2004.00517.x. [DOI] [PubMed] [Google Scholar]