Table 1.
Initial ligand screen.a
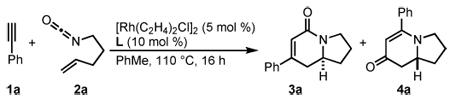 | |||||
|---|---|---|---|---|---|
| entry | L | 3a:4ab | yield (%)c | ee (%)d3a | ee (%)d4a |
| 1 | P(4-MeO-C6H4)3 | 1:1 | <20 | - | - |
| 2 | BINAP | - | NR | - | - |
| 3 | dppb | >20:1 | <5 | - | - |
| 4 | B1 | 1:2.2 | 32 | 5e | 55e |
| 5 | B2 | 1:4.5 | 50 | 45 | 8 |
| 6 | B3 | 1:2.7 | 26 | 5e | 45e |
| 7 | T1 | 1:7.0 | 80 | 83 | 94 |
| 8 | T2 | 1:7.3 | 87 | 89 | 94 |
| 9 | T3 | 1:3.3 | 76 | 90 | 81 |
Reaction conditions: 1 (2 equiv), 2, [Rh(C2H4)2Cl]2 5 mol %, L 10 mol % in PhMe at 110 °C for 16 h.
Ratio determined by 1H NMR of crude reaction.
Combined isolated yield.
Determined by HPLC analysis on a chiral stationary phase.
Opposite enantiomer.
