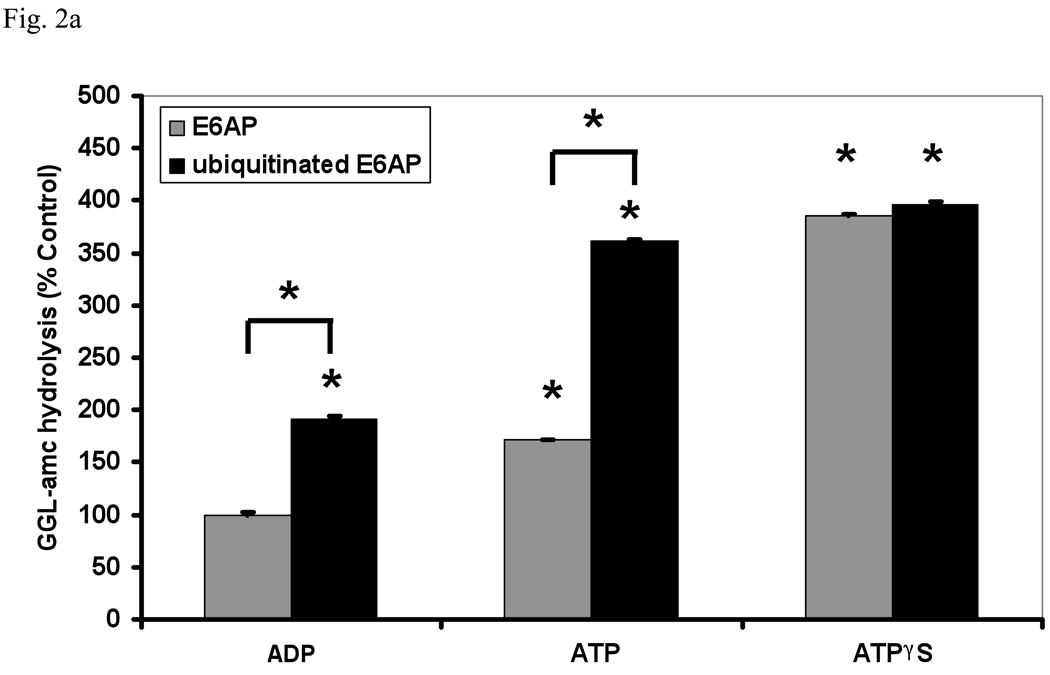
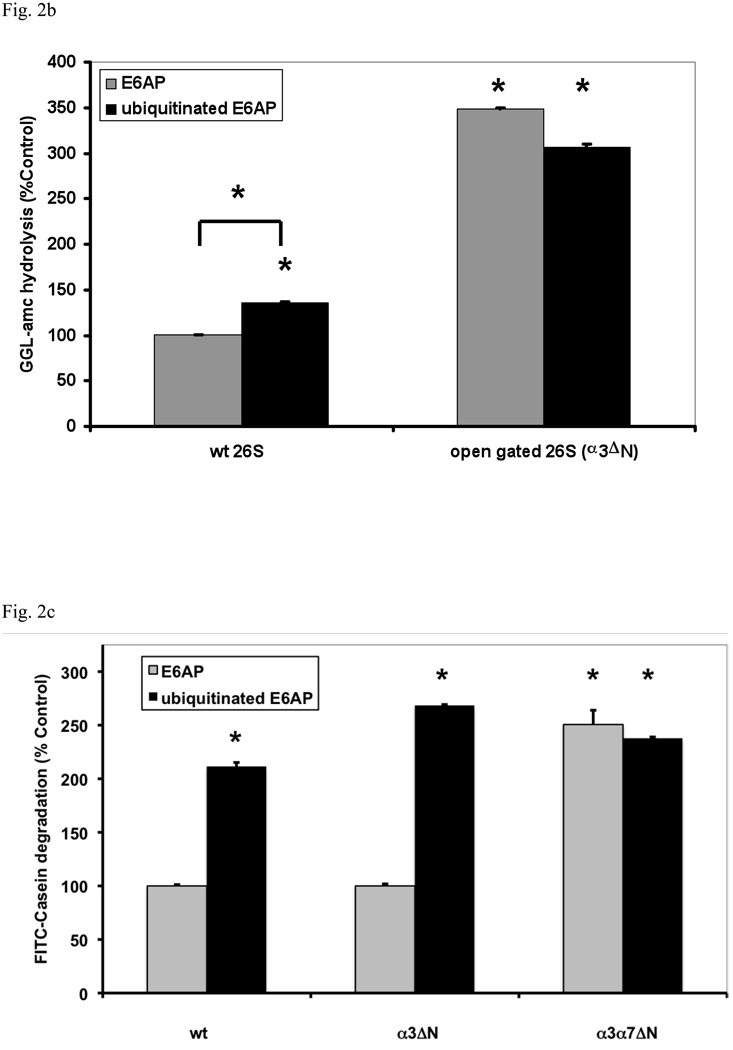
Fig. 2. Ub conjugates stimulate peptide hydrolysis by inducing gate opening in the 20S particle.
(a) Stimulation by Ub conjugates occurs with ADP, which maintains the closed conformation of the gate, and ATP which supports gate opening, but not ATPγS where the open gate conformation is maintained continuously. 26S proteasomes were incubated with E6AP or ubiquitinated E6AP in the presence of ADP, ATP or ATPγS (1 mM). Peptide hydrolysis was measured using GGL-amc. 26S activity in the presence of ADP and E6AP was taken as 100%. The asterisk indicates P< 0.05 difference from the control. (b) Ubiquitinated E6AP increased peptide hydrolysis by 26S proteasomes from wt yeast, but not the open gated mutant (α3ΔN). The proteasomes were purified and equal amounts incubated with E6AP or ubiquitinated E6AP as in Fig. 1. The stimulation observed with wt yeast 26S proteasomes is consistently smaller than with mammalian for Ub conjugates but not for Ub aldehyde (see below). The wt incubated with E6AP was taken as 100%. The asterisk indicates P< 0.05 from wt and E6AP. (c) Ub conjugates increase casein hydrolysis in the wt and α3ΔN open-gated 26S particles but not in the α3α7ΔN 26S proteasomes. FITC-casein degradation was measured as described in Fig. 1. The asterisk indicates P< 0.05 from wt and E6AP treated controls.