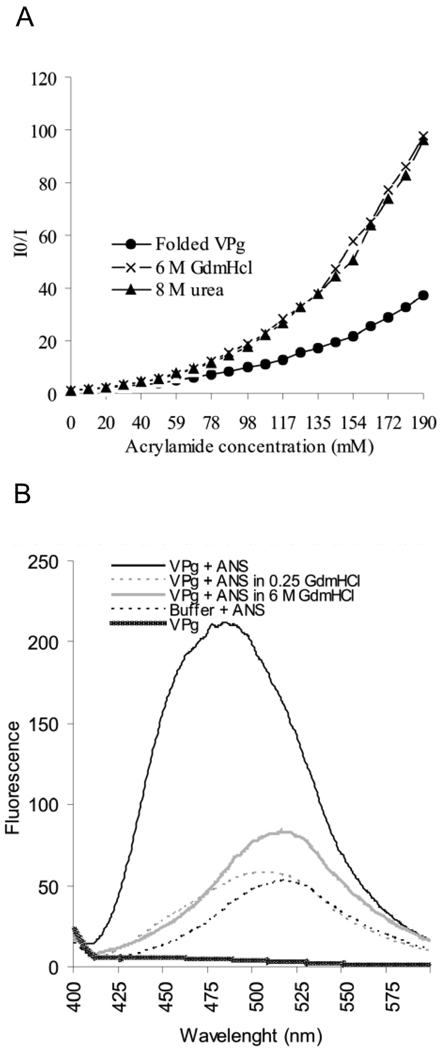
Fig 4.
Fluorescence spectroscopy studies on the compactness and degree of folding of PVA VPg. (A) Intrinsic fluorescence quenching in the presence of increasing acrylamide concentrations was determined for folded and 6M GdmHCl- and 8M urea-denatured samples of VPg. An excitation wavelength of 270 nm was used and the intensity of the emission was monitored between 275–400 nm. I0 indicates the intensity of fluorescence without added acrylamide, and I the intensity in the presence of acrylamide. I0/I ratio was plotted against the increasing acyrlamide concentrations in the Stern-Volmer plot for fluorescence quenching. (B) Fluorescence spectra of folded and denatured VPg was determined after excitation at wavelength 350 nm and by measuring emission between 400–600 nm in the presence and absence of 20µM concentration of ANS. The intensity of fluorescence was plotted against the wavelength. A blue shift of the emission maximum and a 2-fold increase in the intensity with folded VPg indicates the presence of a solvent-accessible hydrophobic core in the protein.