Abstract
Whole body cooling is the current therapy of choice for heatstroke because the therapeutic agents are not available. In this study, we assessed the effects of whole body cooling on several indices of acute lung inflammation and injury which might occur during heatstroke. Anesthetized rats were randomized into the following groups and given (a) no treatment or (b) whole body cooling immediately after onset of heatstroke. As compared with the normothermic controls, the untreated heatstroke rats had higher levels of pleural exudates volume and polymorphonuclear cell numbers, lung myloperoxidase activity and inducible nitric oxide synthase expression, histologic lung injury score, and bronchoalveolar proinflammatory cytokines and glutamate, and PaCO2. In contrast, the values of mean arterial pressure, heart rate, PaO2, pH, and blood HCO3− were all significantly lower during heatstroke. The acute lung inflammation and injury and electrolyte imbalance that occurred during heatstroke were significantly reduced by whole body cooling. In conclusion, we identified heat-induced acute lung inflammation and injury and electrolyte imbalance could be ameliorated by whole body cooling.
1. Introduction
In an anesthetized rat model, elevating core temperature from 36°C to 42°C reduced splanchnic blood flow by 40% and promoted circulatory and intestinal barrier dysfunction [1]. In addition, systemic inflammation, activated coagulation, and renal, hepatic, and brain dysfunctions were noted [2]. Clinically, according to a recent survey, more than three quarters of the studied heatstroke patients developed multiple organ dysfunction, with the most common dysfunction being respiratory failure [3]. A more recent report has also demonstrated that extensive apoptosis as well as inflammation is noted in lung of baboons during heatstroke [4].
Whole body cooling (WBC) is the current therapy of choice for heatstroke because no pharmacologic agent is available [5]. However, the true mechanisms of protection exerted by WBC during heatstroke remain unclear. In an experimental heatstroke model, we have shown that WBC protects against heatstroke by reducing hypoxemia, lactacidemia, acidosis, hypotension, cerebral ischemia and neuronal damage [6].
The objectives of this study are first to further assess whether acute lung inflammation and injury can be induced in rats attendant with heatstroke; second, to ascertain whether WBC therapy is able to attenuate the proposed heatstroke-induced acute lung inflammation and injury. In terms of the 3R concept described by Russell and Burch [7], the reason why we used rats and not other inferior models like mice is that we are used to conduct heatstroke experiments in anesthetized rats [8, 9]. In addition, in performing biochemical and histological verifications, it is better using rats than mice.
2. Materials and Methods
Adult male Sprague-Dawley rats (weighing 238–312 g) were obtained from the Animal Resource Center of the National Science Council of Republic of China. The animals were housed 4 to a cage at an ambient temperature of 22°C ± 1°C, with a 12-h light/dark cycle. Pelleted rat chow and tap water were available ad libitum. The experimental protocol was approved by the Animal Committee of the Chi Mei Foundation Hospital. Animal care and experiments were conducted according to the National Science Council guidelines. They were allowed to become acclimated for ≧1 week. Adequate anesthesia was maintained to abolish the corneal reflex and pain reflex induced by tail-pinching throughout all experiments by an intraperitoneal dose of sodium pentobarbital (60 mg/kg of body weight). Although mechanical ventilation is an integral part of supportive care in acute respiratory distress syndrome, overpressed positive ventilation whether mechanically or manually performed can cause a variety of adverse effects on the lung parenchyma and pulmonary circulation [10]. In the present study, our anesthetized rats were not ventilated at all. At the end of the experiments, control rats and any rats that had survived heatstroke were killed with an overdose of sodium pentobarbital.
The right femoral artery of the rats was cannulated with polyethylene tubing (PE50), under anesthesia, for blood pressure monitoring. Core temperature was monitored continuously by a thermocouple (DR130, Yokogawa, Yamanashiken, Japan) inserted into the rectum, while mean arterial pressure (MAP) and heart rate (HR) were continuously monitored with a pressure transducer and a chart recorder (2107, Gould, Valley view, OH, USA). Arterial blood pH, hematocrit (HCT), PaCO2, PaO2, and HCO3− were measured via a blood gas analyzer (Nova Biochemical, Waltham, MA, USA). The samples were drawn from rats at 42°C and measured in vitro at 37°C. The results presented in this paper were corrected for temperature.
Before induction of heat stress, the core temperature of the anesthetized animals was maintained at about 36°C with a folded heating pad except in the heat stress experiment. Heatstroke was induced by putting the animals in a folded heating pad of 43°C controlled by circulating hot water. The time point in which the MAP dropped from the peak and core temperature over 42°C was arbitrarily taken as the onset of heatstroke [2, 11]. Immediately after this time point (68 minute), the heating pad was removed and the animals were allowed to recover at room temperature (26°C). Therefore, in the following experiments, all heat stressed animals was exposed to 43°C for exactly 68 minute, treated or untreated with WBC, and then allowed to recover at room temperature (26°C). WBC was accomplished by decreasing the blanket temperature from 43°C to 16°C within 2 minute for 20 minute. A group of normothermic rats were exposed to an ambient temperature of 26°C.
The lungs were lavaged by installation of 5 mL of saline at room temperature through a polyethylene tube (2.0 mm in diameter) into the trachea. The first 5 mL of saline installed into the lung were withdrawn and another 5 mL of saline were reinstalled once again. After centrifugation (830 g for 10 minute), the supernatant of first BAL fluid was frozen at −80°C for cytokine assay, whereas the supernatant of second BAL fluid was collected for glutamate determination. BAL fluid was obtained at 88 minute after the initiation of heat stress or at the equivalent time for normothermic controls.
The concentrations of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in BAL fluid were determined using double-antibody sandwich ELISA (R&D systems, Minneapolis, Minn, USA) according to the manufacturer's instructions. Optical densities were read on a plate reader set at 450 nm for TNF-α, IL-1β, and IL-6. The concentrations of cytokines in the samples were calculated from the standard curve multiplied by the dilution factor and were expressed as pg/mL.
For determination of glutamate, aliquots (5 mL) of samples were injected into a CMA600 microdialysis analyzer (Carnegie Medicine, Stockholm, Sweden) for measurement of glutamate.
Animals were killed and their right lungs were immersed in 100% neutral formalin overnight and processed the next day. To assess lung injury, four sections were cut from the lung base toward the apex at 3-mm intervals and stained with hematoxylin and eosin. Ten random fields per animal were read under × 200 magnification by a pathologist who was blinded to the grouping of the rats. According to the grading system of Hong et al. [12], histological variables of acute lung injury examined, each graded from 0 to 3, were (a) intra-alveolar infiltration of neutrophils, (b) interstitial infiltration of neutrophils, (c) perivenous infiltration of neutrophils, (d) pulmonary congestion, and (e) alveolar hemorrhage.
Deparaffinized and rehydrated 4 μm thick sections were treated with 3% H2O2 to block endogenous peroxidase, and incubated with normal blocking serum for 30 minute to block nonspecific staining. Sections were incubated for 1 hour at room temperature, and then overnight at 4°C in a humidity chamber with polyclonal rabbit antimouse iNOS antibody (1 : 500 dilution, Affinity Bioreagent, Golden, CD, USA). After washing in PBS (pH = 7.5), the sections were sequentially incubated with biotinylated goat anti rabbit IgG (1 : 500) and peroxidase-labelled streptavidine (Dako, Santa Barbra, CA, USA). The substrate reaction was carried out using 3-amino-9-ethylcarbazole. The sections were then counterstained with hematoxylin and mounted in an aqueous mounting media. Semiquantitation of the expression of iNOS in lung tissue from rats was performed according to the methods of Furusu et al. [13]. Briefly, the following scale from 0 to 3 was used: 0, no specific staining; 0.5, possibly positive; 1, weakly positive; 2, moderately positive; and 3, strongly positive. Scoring was generally influenced by the extent rather than the intensity of staining.
Myeloperoxidase (MPO) activity, an index of PMNs accumulation, was determined as previously described [14]. Lung tissues, collected at the specified time, were homogenized in a solution containing 0.5% hexa-decyl-trimethyl- ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 minute at 20000 × g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetra-methyl-benzedrine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured by spectrophotometry at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmolmL−1 of peroxide at 37°C and was expressed in ng/mg protein of wet tissue.
The chest was carefully opened and the pleural cavity was washed with 2 mL of saline solution with heparin (5 U mL−1) and indomethacin (10 μgmL−1). The exudates and the washing solution were removed by aspiration and the total volume was measured. Exudates contaminated with blood were discarded. The results were calculated by subtracting the volume injected (2 mL) from the total volume recovered. Cells were counted with the aid of a hemocytometer, and PMN populations were found to contain at least 95% PMN as demonstrated by cytospin and differential stain analysis (vital Trypan Blue stain).
Statistical analysis was carried out using the program SPSS 7.5 (SPSS, Chicago, IL, USA). We assumed that data followed a Gaussian distribution authorizing the use of a one-way analysis to variance with a Tukey post test to analyze the difference between the groups. Wilcoxon's tests were used for evaluation of iNOS immunoreactivity. Wilcoxon's tests convert the scores or values of a variable to rank, require calculation of a sum of the ranks, and provide critical values necessary to test the null hypothesis at a given significant level. These data were presented as “median,” followed by first (Q1) and third (Q3) quartile. P < .05 was considered evidence of statistical significance.
3. Results
The survival time values during heatstroke for untreated rats were decreased from the control values of 476–484 minute (n = 8) to new values of 19–25 minute (n = 8). Resuscitation with WBC decreased core temperature from 42.6°C ± 0.4°C to 39.6°C ± 0.3°C within 20 minute and increased the survival time values (229–283 minute; n = 8) during heatstroke. Normothermic control animals were killed about 480 minute after the initiation of experimentation (or at the end of the experiments).
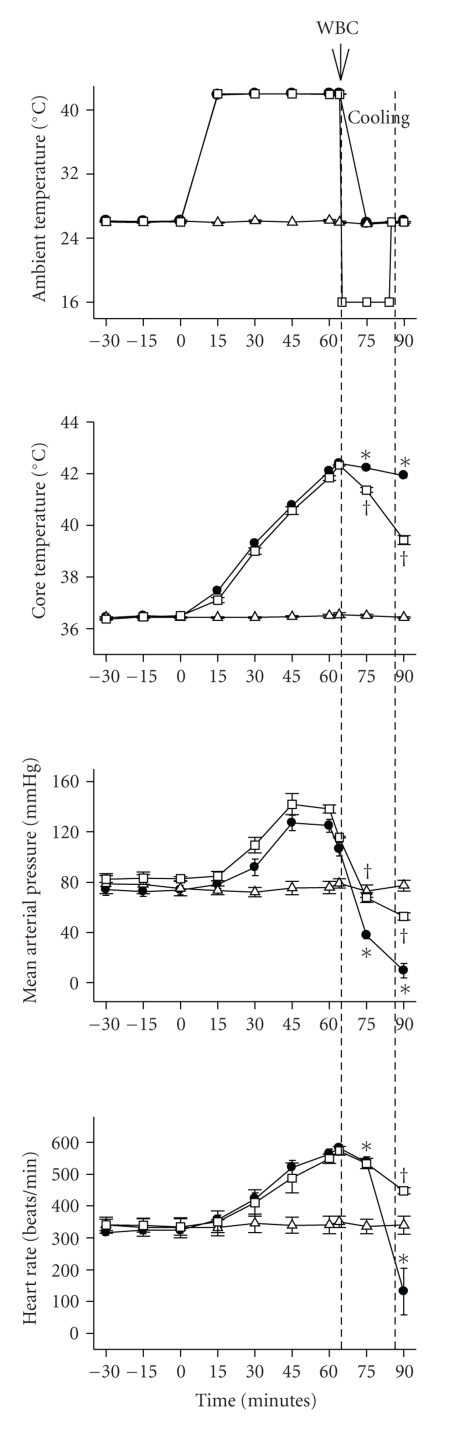
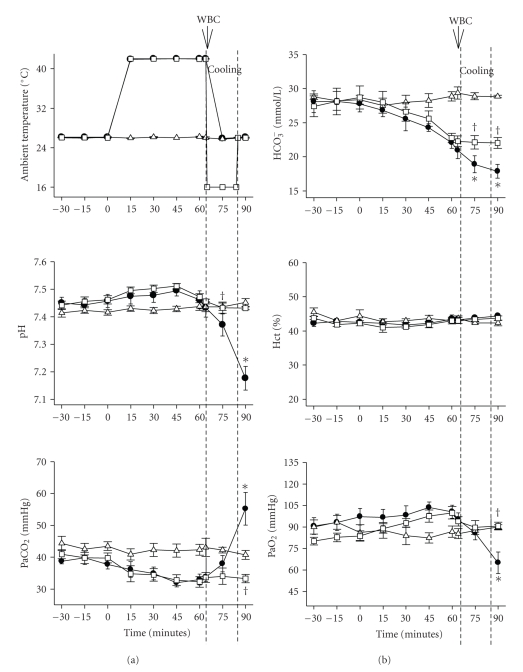
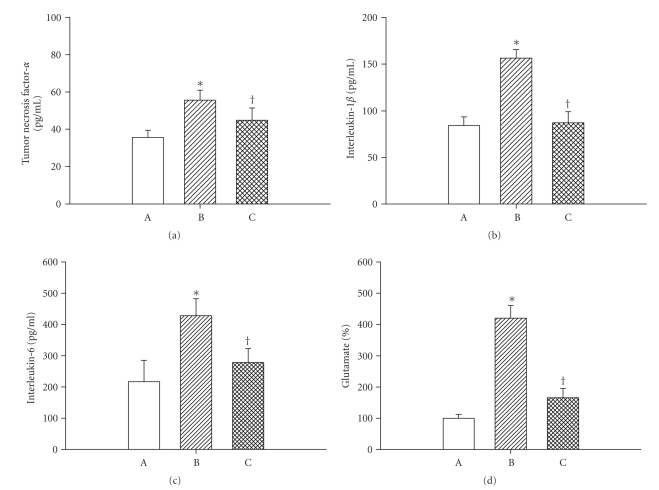
Figures 1, 2, and 3 depict the effects of heat exposure (43°C for 68 minute) on several physiologic and biochemical parameters in heatstroke rats and normothermic controls. In untreated heatstroke group, the core temperature, PaCO2, and BAL fluid levels of tumor necrosis factor-α, interlukin1-β, interleukin-6, and glutamate were all significantly higher at 90 minute after the start of heat exposure than they were for normothermic controls. In contrast, the values for mean arterial pressure, heart rate, pH, PaO2 and HCO3−, were all significantly lower than those of normothermic controls. Resuscitation with WBC immediately at the onset of heatstroke significantly attenuated the heat stress-induced arterial hypotension, bradycardia, hypoxia, acidosis, hyperthermia, and increased BAL fluid levels of porinflammatory cytokines and glutamate.
Figure 1.
Values of ambient temperature, core temperature, mean arterial pressure, and heart rate for normothermic rats (∆), untreated heat stressed rats (●), and heat stressed rats treated with whole body cooling (WBC) for 20 minute (□). Points represent mean ± SEM or 8 rats per group. [*P < .05 in comparison with normothermic control values; †P < .05 in comparison with untreated heat stressed rats]. The duration of WBC is indicated by the dashed line.
Figure 2.
Values of ambient temperature, pH, PaCO2, PaO2, HCO3−, and Hct for normothermic rats (∆), untreated heat stressed rats (●), and heat stressed rats treated with whole body cooling (WBC) for 20 minute (□). Points represent mean ± SEM or 8 rats per group [*P < .05 in comparison with normothermic control; †P < .05 in comparison with untreated heat stressed rats]. The duration of WBC is indicated by the dashed line. The hematocrit is also described.
Figure 3.
Values of bronchoalveolar fluid levels of TNF-α, IL-1β, IL-6, and glutamate for normothermic rats (A; n = 8), untreated heat stressed rats (B; n = 8), heat stressed rats treated with whole body cooling for 20 minute (C; n = 8). *P < .05 in comparison with A; †P < .05 in comparison with B.
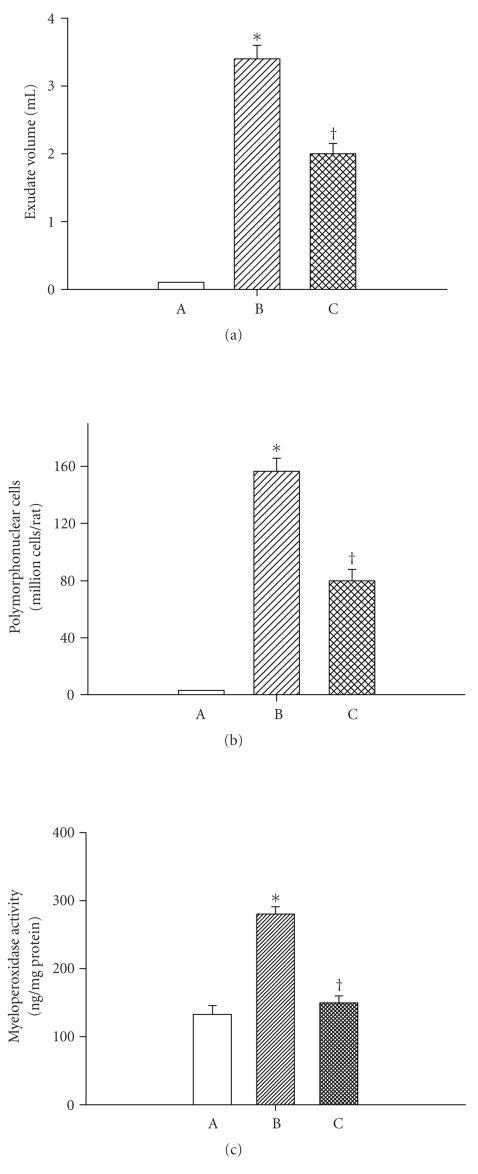
Figures 4 and 5 depict the effects of heat exposure (43°C for 68 minute) on acute pleurisy, lung myeloperoxidase and inducible nitric oxide synthase expression, and acute lung injury score in both heatstroke rats and normothermic controls. In untreated heatstroke group, the values for exudates volume and polymorphonuclear cell numbers in the pleural fluid, the MPO activity in the lung tissues, and histologic acute lung injury score were all significantly higher than those of normothermic controls at 90 minutes after the start of heat exposure. Resuscitation with WBC immediately at the onset of heatstroke significantly attenuated the heat stress-induced acute lung inflammation and injury.
Figure 4.
Values of both exudates volume and amounts of PMNs and myeloperoxidase activity in the lung tissues for normothermic controls (A; n = 8), untreated heat stressed rats (B; n = 8), and heat stressed rats treated with whole body cooling for 20 minute (C; n = 8). *P < .05 in comparison with A; †P < .05 in comparison with B.
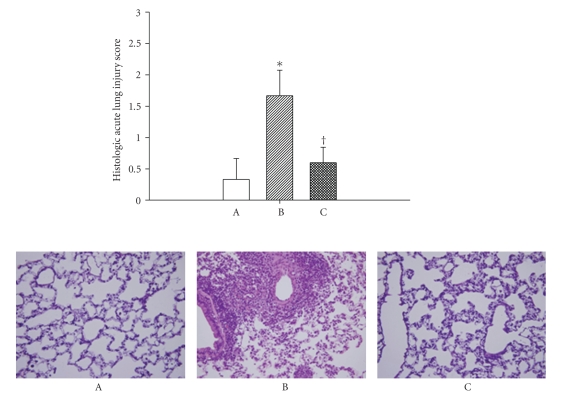
Figure 5.
Values of acute lung injury histologic score (top panel) as well as illustrative lung microscopic picture (bottom panel) for normothermic rats (A; n = 8), untreated heat stressed rats (B; n = 8), and heat stressed rats treated with whole body cooling for 20 minute (C; n = 8). The untreated heat stressed rat displayed advanced alveolar collapse, thicken interstitium in perivascular space, and severe inflammatory cells infiltration in alveolar space and interstitium. The lung pathologic changes that occurred during heat stress were greatly attenuated by whole body cooling. X 400.
In untreated heatstroke rats, the lung iNOS immunoreactivity was significantly greater than that of the normothermic controls (2 (2, 2) versus 0(0, 0)) (P < .05; n = 6). Again, resuscitation with WBC immediately at the onset of heatstroke significantly reduced the heat stress-induced increased lung iNOS immunoreactivity [0 (0, 1)] (P < .05; n = 6). A typical example is depicted in Figure 6.
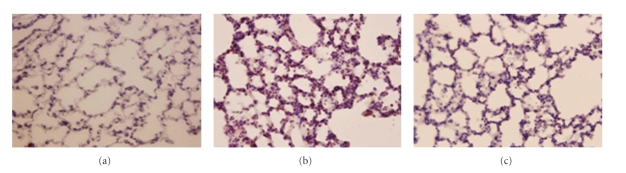
Figure 6.
Photographs showing lung inducible nitric oxide synthase immunoreactivity for a normothermic control (A), an untreated heat stressed rat (B), and a heat stressed rat treated with whole body cooling for 20 minute (C). The increased immunoreactivity of inducible nitric oxide synthase that occurred during heat stress (B) was greatly attenuated by whole body cooling.
4. Discussion
Lipopolysaccharide (LPS) have been administered intraperitoneally, intratracheally as well as intranasally to evoke an acute lung injury in rats [15, 16]. In addition, intraperitoneal administration of LPS resulted in cardiovascular collapse with mild acute lung injury rather than significant hypoxemia and pulmonary edema [17]. In rats, intraperitoneal administration of LPS also caused increased wet/dry ratio, TNF-α concentrations in BAL fluid, and MPO activities in lung tissues when compared to saline solution control group [18]. When rats are exposed to high ambient temperature, hypoxia resulting from splanchnic ischemia induced generation of highly reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the gut that accelerate intestinal mucosal permeability to endotoxin [19]. Endotoxemia could stimulate the production of proinflammatory cytokines, such as interleukin-1β, TNF-α, and interleukin-6 which might trigger systemic inflammation [20]. Immunization against bacterial endotoxin or administration of antibiotics before heat stress markedly reduced mortality in rats [21]. The hypotension associated with heatstroke could be mimicked by systemic administration of interleukin-1β but attenuated by pretreatment of rodents with an antagonist of interleukin-1 receptors [22]. The present study further showed that heatstroke animals displayed increased cellular ischemia markers [6] (e.g., glutamate, lactate-to-pyruvate ratio, and nitric oxide metabolite), proinflammatory cytokines (e.g., interleukin-1β and TNF-α) in BAL fluid, MPO activities and neutrophils infiltration in lung tissues, and lung damage score. The acute lung inflammation and injury that occurred during heatstroke was associated with hyperthermia and arterial hypotension. Putting these observations together, it seems that severe heat stress causes both hyperthermia and splanchnic ischemia which result in endotoxemia, hypotension, and acute lung injury.
The present results, are in part consistent with several investigators. For example, using a conscious, temperature controlled mouse model, it has been shown that maintaining a core temperature at 39°C-40°C rather than at euthermic levels (36.5°C to 37°C) during hyperoxia exposure accelerates lethal pulmonary vascular endothelial injury, reduces the inspired oxygen threshold for lethality, induces expressing of granulocyte-colony stimulating factor, and expands the correlating neutrophils pool [23]. In these same mice, hyperthermia enhances recruitment of neutrophils and changes the histological pattern of lung injury to a neutrophilic interstitial Pneumonitis. In addition, Rice and colleagues [24] have observed that hyperthermia augments neutrophils accumulation and enhances lung injury in experimental Gram-negative bacterial Pneumonia in a mouse model. In anesthetized baboons during heatstroke signaled by hypotension and hyperthermia, extensive apoptosis and inflammation were noted in the lung [4].
The mode of protective action of WBC derived from our previous [6] and present results is apparently multifocal in progressing cascade events in heatstroke reactions; namely, suppressing (a) hyperthermia, (b) hypoxemia, (C) lactacidemia, (d) acidosis, (e) arterial hypotension, (f) endotoxemia, (g) cerebral ischemia and injury, (h) acute lung injury, and (i) animal death. Although our findings support the clinical point of view that generalized hypothermia may be useful for improving outcome in stroke victims [25], hyperthermia may not be the sole cause of heatstroke. It was found that despite WBC, heatstroke was often fatal in humans [25]. Even under the absence of WBC, heatstroke reactions in rodents still could be reversed by various agents or measures [8]. These observations prompted us to think that cardiopulmonary dysfunction as well as electrolytes imbalance is crucial for heatstroke therapy.
Our results are in part consistent with many investigators. For example, hypothermia in rats attenuated the degrees of vascular manifestations and alveolar epithelial injuries induced by injurious ventilation, and preserved the mechanical properties of the lung [26]. Reduction of ventilatory frequency in conjunction with hypothermia attenuated many variables of acute lung injury in rats [12]. Ischemia-reperfusion injury to the rabbit lung can be prevented by hypothermia [27]. Hypothermia and prostaglandin E1 produced synergistic attenuation of ischemia-reperfusion lung injury in rats [28].
There are evidences that proinflammatory cytokines help to propagate the extension of a local or systemic inflammatory process [29]. We confirm here that the inflammatory process (heat-induced pleurisy) leads to a substantial increase in the levels of TNF-α, IL-1β, and IL-6 in the BAL fluids which likely contribute in different capacities to the evaluation of acute inflammation. In fact, it has been shown that the levels of IL-1β in the BAL fluids are elevated in patients with adult respiratory distress syndrome [30]. TNF-α is believed to be an important mediator in the early cascade of endotoxin-induced lung inflammation [31]. In addition, we provide first data to show that heat-induced overproduction of these proinflammatory cytokines in the BAL fluids can be significantly attenuated by WBC therapy.
The inducible isoforms of NOS (iNOS) can be induced by proinflammatory agents, such as lipopolysaccharide, TNF-α, and IL-1β in many cell types [32, 33]. Enhanced formation of NO following the induction of iNOS has been implicated in the pathogenesis of shock and inflammation [32]. Inhibition of iNOS suppresses TNF-α production in the delayed phase of allergic encephalomyelitis [34], IL-1 production in arthritis [35], and production of nitrite/nitrate, TNF-α, and IL-1β in acute lung inflammation (due to injection of carrageenan into the pleural cavity) [36]. These observations tend to indicate that iNOS-derived NO plays a role in the development of the inflammatory response by altering key components of the inflammatory cascade. In fact, both plasma or brain NO levels have been elevated in heatstroke patients [37] and rats [1, 38]. Aminoguanidine, an iNOS inhibitor, improves heat tolerance in rats by preserving splanchnic blood [1] and cerebral blood flow [38]. The present results further show that WBC therapy, like iNOS inhibitors, is able to attenuate both the acute lung injury and the lung immunoreactivity of iNOS during heat stress. The results are supported by Scumpia et al. [39] showing that WBC attenuates iNOS expressing in the lungs of endotoxemic rats.
Excessive activation of N-methyl-D-aspartate (NMDA) receptors in the lung has been found to be involved in genesis of acute lung inflammation during the adult respiratory distress syndrome [40] and hypoxia [41]. Excessive accumulation of glutamate has also been shown in primary or secondary ischemic brain tissues [6]. Our current findings demonstrate that the excessive concentrations of glutamate in the BAL fluids during heat stress are significantly reduced by WBC therapy. Sakuma et al. have also demonstrated that the ischemia-reperfusion lung injury in rabbits can be reduced by hypothermia [27]. Thus, we infer that WBC may cause attenuation of heat-induced lung inflammation and injury via reducing glutamate overproduction.
In summary, our present results demonstrate that WBC improves the outcomes of heatstroke by decreasing cardiopulmonary dysfunction and electrolytes imbalance in a rat model. Our previous results [6] have also demonstrated that WBC or brain cooling improves the outcomes of heatstroke by attenuating microvascular injury, thrombosis, inflammation, and apoptosis in several vital organs [6, 42, 43]. Together, both of our previous and present results suggest that WBC may improve outcomes of heatstroke via attenuating multiple organs (including lungs) dysfunction or failure.
Acknowledgment
This study was supported by the National Science Council of the Republic of China (Taipei, Taiwan), Grants NSC 96-2314-B-384-002, NSC96-2314-B-384-003-MY3, NSC96-2320-B-218-001-MY2, and NSC96-234-B-384-006-MY3.
References
- 1.Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. American Journal of Physiology. 2001;280(2):H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- 2.Chen S-H, Chang F-M, Tsai Y-C, Huang K-F, Lin M-T. Resuscitation from experimental heatstroke by transplantation of human umbilical cord blood cells. Critical Care Medicine. 2005;33(6):1377–1383. doi: 10.1097/01.ccm.0000165966.28936.89. [DOI] [PubMed] [Google Scholar]
- 3.Varghese GM, John G, Thomas K, Abraham OC, Mathai D. Predictors of multi-organ dysfunction in heatstroke. Emergency Medicine Journal. 2005;22(3):185–187. doi: 10.1136/emj.2003.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts GT, Ghebeh H, Chishti MA, et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(6):1130–1136. doi: 10.1161/ATVBAHA.107.158709. [DOI] [PubMed] [Google Scholar]
- 5.Simon HB. Hyperthermia and heatstroke. Hospital Practice. 1994;29(8):65–80. doi: 10.1080/21548331.1994.11443062. [DOI] [PubMed] [Google Scholar]
- 6.Chou YT, Lai ST, Lee CC, Lin MT. Hypothermia attenuates circulatory shock and cerebral ischemia in experimental heatstroke. Shock. 2003;19(4):388–393. doi: 10.1097/00024382-200304000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Russell W, Burch R. The Principles of Humane Experimental Technique. London, UK: Methuen; 1959. [Google Scholar]
- 8.Chang C-K, Chang C-P, Chiu W-T, Lin M-T. Prevention and repair of circulatory shock and cerebral ischemia/injury by various agents in experimental heatstroke. Current Medicinal Chemistry. 2006;13(26):3145–3154. doi: 10.2174/092986706778742945. [DOI] [PubMed] [Google Scholar]
- 9.Chang C-K, Chang C-P, Liu S-Y, Lin M-T. Oxidative stress and ischemic injuries in heat stroke. Progress in Brain Research. 2007;162:525–546. doi: 10.1016/S0079-6123(06)62025-6. [DOI] [PubMed] [Google Scholar]
- 10.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. American Journal of Respiratory and Critical Care Medicine. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 11.Chen S-H, Chang F-M, Chang H-K, Chen W-C, Huang K-F, Lin M-T. Human umbilical cord blood-derived CD34+ cells cause attenuation of multiorgan dysfunction during experimental heatstroke. Shock. 2007;27(6):663–671. doi: 10.1097/01.shk.0000248593.71388.40. [DOI] [PubMed] [Google Scholar]
- 12.Hong S-B, Koh Y, Lee I-C, et al. Induced hypothermia as a new approach to lung rest for the acutely injured lung. Critical Care Medicine. 2005;33(9):2049–2055. doi: 10.1097/01.ccm.0000178186.37167.53. [DOI] [PubMed] [Google Scholar]
- 13.Furusu A, Miyazaki M, Abe K, et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney International. 1998;53(6):1760–1768. doi: 10.1046/j.1523-1755.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 14.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. Journal of Pharmacological Methods. 1985;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 15.Rinaldo JE, Dauber JH, Christman J, Rogers RM. Neutrophil alveolitis following endotoxemia. Enhancement by previous exposure to hyperoxia. American Review of Respiratory Disease. 1984;130(6):1065–1071. doi: 10.1164/arrd.1984.130.6.1065. [DOI] [PubMed] [Google Scholar]
- 16.Wheeldon EB, Walker ME, Murphy DJ, Turner CR. Intratracheal aerosolization of endotoxin in the rat: a model of the adult respiratory distress syndrome (ARDS) Laboratory Animals. 1992;26(1):29–37. doi: 10.1258/002367792780809020. [DOI] [PubMed] [Google Scholar]
- 17.Lu M-Y, Kang B-H, Wan F-J, Chen C-S, Huang K-L. Hyperbaric oxygen attenuates lipopolysaccharide-induced acute lung injury. Intensive Care Medicine. 2002;28(5):636–641. doi: 10.1007/s00134-002-1262-1. [DOI] [PubMed] [Google Scholar]
- 18.Chu S-J, Perng W-C, Hung C-M, Chang D-M, Lin S-H, Huang K-L. Effects of various body temperatures after lipopolysaccharide-induced lung injury in rats. Chest. 2005;128(1):327–336. doi: 10.1378/chest.128.1.327. [DOI] [PubMed] [Google Scholar]
- 19.Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. American Journal of Physiology. 1999;276(5):G1195–G1203. doi: 10.1152/ajpgi.1999.276.5.G1195. [DOI] [PubMed] [Google Scholar]
- 20.Doran JE. Biological effects of endotoxin. In: Kraft CH, editor. Gut-Derived Infectious Toxic Shock. Current Studies in Hematology and Blood Transfusion. Vol. 59. Basel, Switzerland: karger; 1992. pp. 66–99. [DOI] [PubMed] [Google Scholar]
- 21.Butkow N, Mitchell D, Laburn H, et al. Heat stroke and endotoxaemia in rabbits. In: Hales JRS, editor. Thermal Physiology. New York, NY, USA: Raven; 1984. pp. 511–514. [Google Scholar]
- 22.Shen K-H, Chang C-K, Lin M-T, Chang C-P. Interleukin-1 receptor antagonist restores homeostatic function and limits multiorgan damage in heatstroke. European Journal of Applied Physiology. 2008;103(5):561–568. doi: 10.1007/s00421-008-0755-1. [DOI] [PubMed] [Google Scholar]
- 23.Hasday JD, Garrison A, Singh IS, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. American Journal of Pathology. 2003;162(6):2005–2017. doi: 10.1016/S0002-9440(10)64333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice P, Martin E, He J-R, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. Journal of Immunology. 2005;174(6):3676–3685. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 25.Simon HB. Hyperthermia. The New England Journal of Medicine. 1993;329(7):483–487. doi: 10.1056/NEJM199308123290708. [DOI] [PubMed] [Google Scholar]
- 26.Lim C-M, Hong S-B, Koh Y, et al. Hypothermia attenuates vascular manifestations of ventilator-induced lung injury in rats. Lung. 2003;181(1):23–34. doi: 10.1007/s00408-002-0111-x. [DOI] [PubMed] [Google Scholar]
- 27.Sakuma T, Takahashi K, Ohya N, et al. Ischemia-reperfusion lung injury in rabbits: mechanisms of injury and protection. American Journal of Physiology. 1999;276(1):L137–L145. doi: 10.1152/ajplung.1999.276.1.L137. [DOI] [PubMed] [Google Scholar]
- 28.Chiang C-H, Wu K, Yu C-P, Yan H-C, Perng W-C, Wu C-P. Hypothermia and prostaglandin E1 produce synergistic attenuation of ischemia-reperfusion lung injury. American Journal of Respiratory and Critical Care Medicine. 1999;160(4):1319–1323. doi: 10.1164/ajrccm.160.4.9811079. [DOI] [PubMed] [Google Scholar]
- 29.McDonald MC, Izumi M, Cuzzocrea S, Thiemermann C. A novel, potent and selective inhibitor of the activity of inducible nitric oxide synthase (GW274150) reduces the organ injury in hemorrhagic shock. Journal of Physiology and Pharmacology. 2002;53(4, part 1):555–569. [PubMed] [Google Scholar]
- 30.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. American Journal of Respiratory and Critical Care Medicine. 1996;153(6, part 1):1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 31.Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. American Journal of Respiratory Cell and Molecular Biology. 1994;10(2):148–153. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]
- 32.Nathan C. Nitric oxide as a secretory product of mammalian cells. The FASEB Journal. 1992;6(12):3051–3064. [PubMed] [Google Scholar]
- 33.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochemical Pharmacology. 1996;51(4):383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 34.Anggard EE. Endogenous nitrates—implications for treatment and prevention. European Heart Journal. 1991;12(supplement A):5–8. [PubMed] [Google Scholar]
- 35.Parker JO. Nitrate therapy in stable angina pectoris. The New England Journal of Medicine. 1987;316(26):1635–1642. doi: 10.1056/NEJM198706253162606. [DOI] [PubMed] [Google Scholar]
- 36.Dugo L, Marzocco S, Mazzon E, et al. Effects of GW274150, a novel and selective inhibitor of iNOS activity, in acute lung inflammation. British Journal of Pharmacology. 2004;141(6):979–987. doi: 10.1038/sj.bjp.0705683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzeer AH, Al-Arifi A, Warsy AS, Ansari Z, Zhang H, Vincent J-L. Nitric oxide production is enhanced in patients with heat stroke. Intensive Care Medicine. 1999;25(1):58–62. doi: 10.1007/s001340050787. [DOI] [PubMed] [Google Scholar]
- 38.Chang C-P, Lee C-C, Chen S-H, Lin M-T. Aminoguanidine protects against intracranial hypertension and cerebral ischemic injury in experimental heatstroke. Journal of Pharmacological Sciences. 2004;95(1):56–64. doi: 10.1254/jphs.95.56. [DOI] [PubMed] [Google Scholar]
- 39.Scumpia PO, Sarcia PJ, DeMarco VG, Stevens BR, Skimming JW. Hypothermia attenuates iNOS, CAT-1, CAT-2, and nitric oxide expression in lungs of endotoxemic rats. American Journal of Physiology. 2002;283(6):L1231–L1238. doi: 10.1152/ajplung.00102.2002. [DOI] [PubMed] [Google Scholar]
- 40.Said SI, Berisha HI, Pakbaz H. Excitotoxicity in the lung: N-methyl-D-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(10):4688–4692. doi: 10.1073/pnas.93.10.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang F, Yue S, Luo Z, et al. Role of N-methyl-D-aspartate receptor in hyperoxia-induced lung injury. Pediatric Pulmonology. 2005;40(5):437–444. doi: 10.1002/ppul.20299. [DOI] [PubMed] [Google Scholar]
- 42.Hsu S-F, Niu K-C, Lin C-L, Lin M-T. Brain cooling causes attenuation of cerebral oxidative stress, systemic inflammation, activated coagulation, and tissue ischemia/injury during heatstroke. Shock. 2006;26(2):210–220. doi: 10.1097/01.shk.0000223124.49265.10. [DOI] [PubMed] [Google Scholar]
- 43.Hsiao S-H, Chang C-P, Chiu T-H, Lin M-T. Resuscitation from experimental heatstroke by brain cooling therapy. Resuscitation. 2007;73(3):437–445. doi: 10.1016/j.resuscitation.2006.11.003. [DOI] [PubMed] [Google Scholar]