Abstract
Objective
The purpose of this study was to analyze risk factors that are associated with intracranial lesion, and to propose criteria for classification of mild head injury (MHI), and appropriate treatment guidelines.
Methods
The study was based on 898 patients who were admitted to our hospital with Glasgow Coma Scale (GCS) score of 13 to 15 between 2003 and 2007. The patients' initial computerized tomography (CT) findings were reviewed and clinical findings that were associated with intracranial lesions were analyzed.
Results
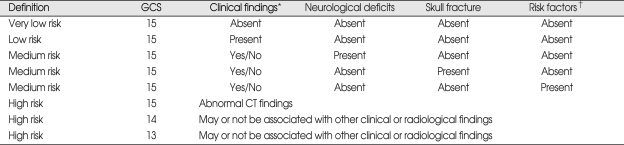
GCS score, loss of consciousness (LOC), age and skull fracture were identified as independent risk factors for intracranial lesions. Based on the data analysed in this study, MHI patients were divided into four subgroups : very low risk MHI patients are those with a GCS score of 15 and without a history of LOC or headache; low risk MHI patients have a GCS score of 15 and with LOC and/or headache; medium risk MHI patients are those with a GCS score of 15 and with a skull fracture, neurological deficits or with one or more of the risk factors; high risk MHI patients are those with a GCS score of 15 with abnormal CT findings and GCS score of 14 and 13.
Conclusion
A more detailed classification of MHI based on brain CT scan findings and clinical risk factors can potentially improve patient diagnosis. In light of our findings, high risk MHI patients should be admitted and treated in same manner as those with moderate head injury.
Keywords: Mild head injury, CT scan findings, Risk factors, Classification, Treatment guideline
INTRODUCTION
The term 'minor head injury' was first defined by Rimel et al.31) in 1981 as head trauma with Glasgow Coma Scale (GCS) score40) of 13-15 on admission, a loss of consciousness (LOC) of less than 20 minutes who were admitted to hospital for less than 48 hours. Generally, mild head injury (MHI) leads to quick recovery without complications and doesn't result in mortality. However, among patients who were diagnosed with MHI, there are instances of patients developing life-threatening complications or long term sequelae-rendering the neurosurgeon unable to respond effectively, given current classification of MHI.
To address this issue, several authors have proposed to redefine MHI using various approaches7,9,12-15,36,37). The rationale behind sub-grouping MHI is to identify risk factors and screen those patients with high risk for acute intracranial lesions, which can adversely affect the patient prognosis. Unfortunately, there exists no consensus regarding such classification.
The main objective of this study is to propose criteria for classifying a patient with MHI to analyze the importance of clinical findings in the detection of patients at risk for intracranial lesions after MHI.
MATERIALS AND METHODS
This study was conducted retrospectively on clinical records of 898 MHI patients who were admitted to our hospital between January 2003 and December 2007. Patients under 16 years old, patients with penetrating cranial trauma and hospital admission of less than 12 hours were excluded. MHI was defined as a GCS score of 13 to 15 at the time of admission, with or without LOC. Since the GCS score may vary depending on the person performing the assessment, our study used the initial GCS score given at the time of admission by the attending neurosurgeon for consistency. Demographic and clinical data were retrospectively collected in all cases. Symptoms, clinical signs, and previous medical conditions considered to be risk factors for developing intracranial lesions after MHI were checked and Skull X-ray and CT scan findings were reviewed for all patients. Abnormal CT scan findings were defined as the following; 1) all intracranial post-traumatic hematoma or contusion, 2) depressed fractures, 3) traumatic subarachnoid hemorrhage, and 4) pneumocephalus. A CT scan demonstrating an acute intracranial lesion was defined as an abnormal CT finding, therefore isolated cases of linear skull fracture and initial diagnosis chronic subdural hematoma were not considered as abnormal CT findings. When the patient displayed neurological deterioration or worsening symptoms, CT scan was repeated. Clinical deterioration was defined as an increase in a preexisting neurological deficit, development of a new focal sign, appearance of pupillary abnormality or a 2 point decrease in GCS score. Patient outcome was assessed 6 months post-injury using the Glasgow Outcome Scale. For outpatients, follow up records were reviewed. For the other patients, prognosis was determined by telephone interviews. Chi-square test and the logistic regression model were used for the analysis. We accepted p values of less than 0.05 as significant for all measures.
RESULTS
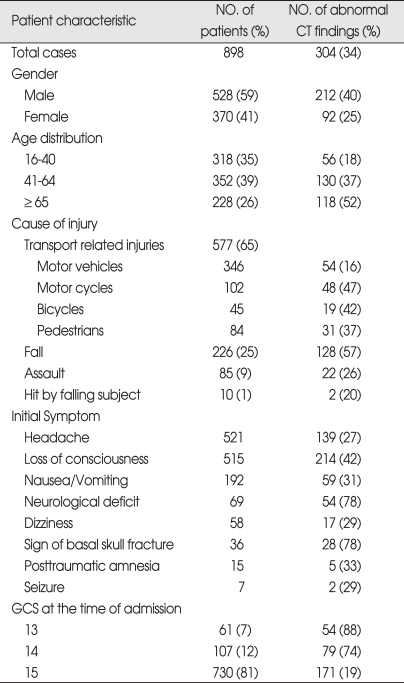
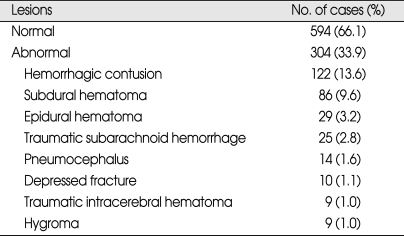
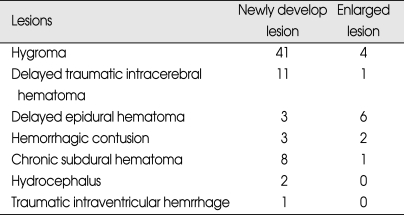
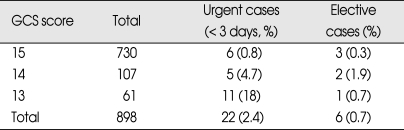
During the 5 year period between 2003 and 2007, 898 patients were included in the study. Of the 898 patients, 528 patients were male and 370 patients were female (ratio 1.4 : 1). The patients ranged in age between 16 and 94 years old and the average age were 49.3 ± 21.7 years. Patients were classified into three age groups for analysis; 16-40 years old, 41-64 years old, and over 65 years old. 352 (39%) patients were 41-64 years old. Among male patients, higher frequency of abnormal CT scan findings was observed, especially in the over 65 years old group compared to the 16-40 years old group. At the time of admission, 730 patients were assigned GCS score of 15, which was the most prevalent score, followed by 107 patients with GCS score of 14 and 61 people with GSC score of 13. Incidences of abnormal CT scan findings were 88% in GCS score of 13 group and 74% in GCS score of 14 group, and 19% in GCS score of 15 group, demonstrating higher incidences of abnormal CT scan finding in the GCS score 13 group. The most common symptom reported by the patients was headache, which was reported by 521 patients, followed by nausea and vomiting. Five-hundred-and-fifteen patients reported LOC, and abnormal CT scan findings were observed in 42% of these patients. Transport-related injuries were the most common causes of injury, accounting for 577 out of the 898 cases, followed by falls and other causes. Among the causes of injuries, fall and motorcycle accidents resulted in higher incidences of abnormal CT scan findings than other causes (Table 1). Skull fractures were observed in 158 patients by skull X-ray and CT scan, and linear skull fracture was the most prevalent type. Of these, 138 patients displayed fracture at the time of admission displayed abnormal CT scan findings, which was higher than patients without skull fracture (Table 2). Initial CT scan showed 594 cases of normal and 304 cases of abnormal findings. Among patients with intracranial lesions, hemorrhagic contusion was the most prevalent in 122 patients, followed by subdural hematoma (SDH) and epidural hematoma (EDH) (Table 3). Eighty-three patients showing newly developed lesions and enlarged pre-existing lesions were assessed by repeat CT scan. Of those, hygroma was the most common, found in 45 patients, followed by delayed traumatic intracerebral hematoma (DTICH) and delayed epidural hematoma (DEDH). Hygroma and DTICH were often found in patients with newly developed lesion, while DEDH was observed in patients with enlarged pre-existing lesion (Table 4). Generally, hygroma cases were from repeat CT scan of patients with headaches, and they were diagnosed within 7 days of the initial injury. DTICH and DEDH were generally observed in repeat CT scan of patients showing neurological deterioration. Twelve patients required repeat CT scan within 24 hours, and of those, 6 patients showed DTICH and 5 patients showed EDH.
Table 1.
Summary of the patient characteristics
CT : computerized tomography, GCS: Glasgow Coma Scale
Table 2.
Incidence of abnormal CT findings in patients with skull fracture
CT : computerized tomography
Table 3.
Initial CT findings
CT : computerized tomography
Table 4.
Analysis of delayed lesions detected from repeat CT scans
CT : computerized tomography
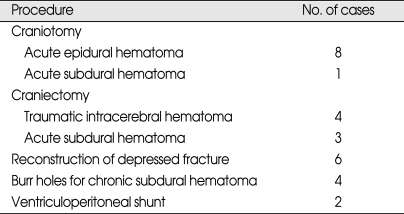
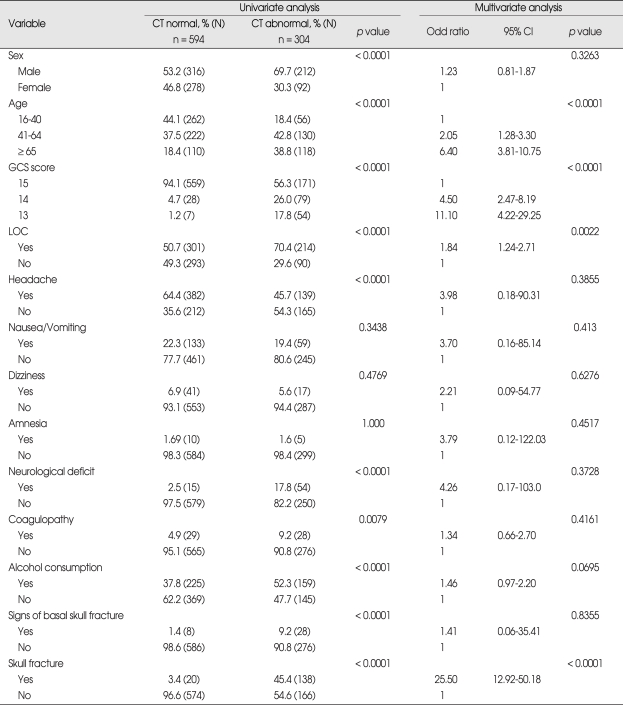
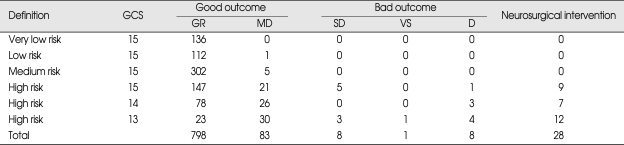
Over the study period, 28 patients received neurosurgical interventions (Table 5). Twenty-two (2.4%) of these required surgery within first third day of injury, and theirs GCS scores on admission were as follows; 11 patients with GCS score 13, 5 patients with GCS score 14, and 6 patients with GCS score 15. Six patients received elective surgery after follow up assessments revealed chronic subdural hematoma and hydrocephalus (Table 6). Results of the uni- and multivariate analyses that relate to clinical findings to the presence of abnormal CT findings are shown in Table 7. Ten variables showed statistically significant relationships with abnormal CT findings in the univariate analysis. Four remained independent risk factors in the multivariate analysis. These were patient age greater than 65 years [odds ratio (OR) 6.40, 95% confidence interval (CI) 3.81-10.75), GCS score (OR 11.10, 95% CI 4.22-29.25), LOC (OR 1.84, 95% CI 1.24-2.71) and skull fracture (OR 25.5, 95% CI 12.92-50.18). Based on these findings, we further classified the existing MHI patients into subgroups. Patients with GCS score of 15 were classified into very low risk, low risk and medium risk MHI. GCS score of 15 with abnormal CT scan findings and GCS score of 14 and 13 were classified as high risk MHI (Table 8). All patients in the very low, low and medium risk MHI displayed favorable outcomes while 17 patients in the high risk MHI displayed adverse outcomes, including eight deaths, and these differences were statistically significant (p < 0.01). In addition, all twenty-eight patients who required surgical treatments were from the high risk group (Table 9). Finally, of the 898 patients in the study, only 17 patients (2%) showed adverse outcomes, a result that is consistent with other previous studies, but the mortality rate of 0.9% was very low.
Table 5.
Summary of operative procedures required in the 28 patients
Table 6.
Association between neurosurgical intervention and GCS score at the admission
GCS : Glasgow Coma Scale
Table 7.
Results of univariate and multivariate analyses relating the clinical variable to the presence of abnormal CT scan
CI : confidence interval, CT : computerized tomography, GCS : Glasgow Coma Scale, LOC : Loss of consciousness
Table 8.
Level of risk for intracranial lesions in mild head injured patients
*One or more loss of consciousness and headache, †Coagulopathy, alcohol consumption, and age over 65 years. GCS : Glasgow Coma Scale
Table 9.
Outcome at 6 months and management results using the new classification of mild head injury
p < 0.01. GCS : Glasgow Coma Scale, GR : good recovery, MD : moderate disability, SD : severe disability, VS : vegetative state, D : death
DISCUSSION
Rimel et al.31) first classified the severity of head injuries based on GCS score. MHI corresponds to GCS score of between 13 and 15. The majority of MHI patients recover without requiring specific treatments, but a small, significant proportion of patients develop serious complications that require urgent neurosurgical care. Accurate evaluation and treatment of patients who are diagnosed with MHI is one of the most important factors that can reduce morbidity and death association with head injury18). To address this issue, several authors have proposed to re-defined MHI using various methods. Williams et al.42) sub-classified MHI into complicated and uncomplicated MHI based on radiological findings. Hsiang et al.12) proposed high-risk MHI subgroup based on GCS score and CT findings, while Servadei et al.35) subgrouped patients with GCS of 14 or 15 into three levels of risk groups. On the other hand, some authors proposed to limit MHI classifica-tion to GCS of 14 to 15 while classifying patients with GCS score of 13 as "moderate head injury" due to the high incidences of intracranial lesion in this group.13,38,42). In our study, we observed incidences of abnormal CT scans in 88% of patients with GCS score of 13, which was markedly higher than 19% observed in patients with GCS score of 15. These results indicate that patients with GCS score of 13 have comparable level of intracranial lesions as moderate head injury patients. Since the GCS score was the most widely used parameter for grading the clinical severity of head injuries, we defined MHI as GSC score of 13-15. However, the term 'mild' can be a misleading label for patients with GCS score of 13, and thus a more detailed classification is needed to distinguish the risk levels.
CT scans are important in determining the presence of intracranial abnormalities in MHI patients and numerous criteria has been proposed to help determine when CT scans should be performed. The National Institute for Health and Clinical Excellence (NICE) guideline for CT imaging included GCS score of 14, signs of basal skull fracture, neurologic deficit, vomiting, amnesia before impact greater than 30 minutes, posttraumatic seizures, coagulopathy, dangerous mechanism, age greater than 64 years43), while another guideline from the Neurotraumatology Committee of the World Federation of Neurosurgical Societies (NCWFNS) proposed GCS score of 14, suspected skull fracture, neurologic deficit, vomiting, amnesia, LOC, hea-dache, history of seizures, previous neurosurgery, alcohol or drug abuse, coagulopathy35). Based on our study, we recommend that CT scans be performed to all cases of head and facial injuries, to assess the severity of the injury. Since adverse outcomes are very rare in MHI patients with normal initial CT scan1), ruling out the chances of adverse outcome by CT scans is helpful in assessing patient risk-levels. With respect to the timing of the CT scans, there are no clear guidelines on how soon a CT scan should be performed after the patient is admitted. In clinical practice, early CT scan probably means anytime between around 30 minutes up to several hours after injury1). Since the initial CT scan is a good indication of the patient outcome, repeat CT scans were performed only for patients with clinical deterioration or with new clinical symptoms4). Incidences of abnormal CT findings differed widely between studies, from 4-34%7,11,37). These differences are due to the varying definition of MHI used by different groups. For example, Rimel et al.31) has used GCS scores between 13 and 15 to define MHI, while Stein and Ross37) used GCS scores of 14 or 15 to classify MHI. Hsiang et al.12) used both GCS score and radiological findings to define MHI and Master et al.22) defined MHI based on clinical symptoms. In addition, significant discrepancies may happen between neurosurgeons and other physicians or paramedical professionals regarding interpretation during assessment of GCS scores21). In our study, the incidences of abnormal CT findings were higher than previously reported, due to the method of classification (inclusion of GCS score 13) and may also be due to higher incidences of injuries from falls and larger proportion of seniors in our patient population. Previous studies have reported that among intracranial lesions, 4-28% were EDH, 10-31% were SDH and 37-69% were contusion and intracerebral hematoma7,9,12,19,27,38). Our study found that hemorrhagic contusion was the most prevalent, at 14% of the total intracranial lesions, followed by SDH and EDH. Whether or not MHI patients need to be assessed by skull radiography is still debated7,12,20,22,24,36). Since skull fracture can be accompanied by intracranial hematoma7,24-26,33), when CT scan is not available, skull radiography is required to confirm the presence of fracture. Risk factors associated with incidences of intracranial lesion can lead to more effective treatment of head injuries. Numerous groups have proposed risk factors, such as age, gender, cause of injury, GCS score, focal neurological deficiencies, headache, vomiting, nausea, seizure, loss of consciousness, post-traumatic amnesia, skull fracture, signs of basal skull fracture, coagulopathy, alcohol consumption, seizure, and previous neurosurgical procedure3,6,11-15,20,24-26,28,39,41). Among these, age (> 65 years old), GCS score (< 15), skull fracture, pedestrian victims of traffic accident and victims of assault and focal neurological deficiencies have been accepted clinical risk factors for the development of intracranial lesions following MHI.
In our study, the following factors were shown to have statistically significant relationship with abnormal CT findings when assessed in univariate statistical analysis; GCS score, LOC, headache, gender, age, coagulopathy, alcohol consumption, neurological deficit, signs of basal skull fracture, and skull fracture. However, multivariate analysis revealed that only age, GCS score, LOC and skull fractures showed statistically significant value in predicting abnormal CT findings. Based on these findings, we further classified the existing MHI patients into four subgroups. Patients with GCS score of 15 were classified into very low and low and medium risk. GCS score of 15 with abnormal CT findings and GCS score 14 and 13 were classified as high risk. All patients in the very low and low and medium risk group displayed favorable outcomes while 17 patients in the high risk group displayed adverse outcomes, including 8 deaths, and these differences were statistically significant (Table 8) (p < 0.01). Based on the current finding, we propose a guideline for treating MHI patients based on their risks for intracranial lesion development. An accurate neurological examination based on the GCS score and pupil size and response, motor power and vital signs must be performed. Clinical variables should also be checked for patients as well as CT scans that need to be performed in all patients with MHI. Very low and low risk group can be allowed to go home with written instruction about symp-toms that can occur which would prompt a return to the hospital. Medium risk patients should be admitted to the hospital for at least 2 or 3 days. High risk group patients should be admitted to an intensive care unit and treated as a moderate head injury.
Previous studies have reported that 0.6-2.4% of MHI patients required surgical treatments7,16,24,26). Consistent with these findings, our study has shown that among 898 patients, 22 (2.4%) required surgical treatments within 3 days of being admitted. Six other patients received elective surgery after follow up assessments revealed chronic subdural hematoma and hydrocephalus. When MHI patients develop intracranial hematoma, several issues must be considered in deciding the course of treatment. Whether to treat the patient surgically or by conservative treatment, the need for additional CT scan assessment and the timing of surgical treatments are some of the considerations that must be addressed. We propose a guideline for treating intracranial hematoma in MHI patients as followings. For epidural hematoma, surgery should be performed in case of significant mass effect, focal neurologic deficit, or coma. In the absence of clinical symptoms, hematoma with a thickness of less than 15 mm5,29,34), a volume of less than 30 cc4) and a midline-shift of less than 5 mm5,17,29) can be treated with conservative treatments. Presence of a heterogeneous clot or lucent swirl density or air within high density areas in CT scans require close observation, since these signs are indications of active bleeding and can lead to hematoma enlargement10,44). Guidelines for conservative treatment of epidural hematoma must also include assessments to determine if repeat CT is needed in the first 12 hours after injury30). Guidelines for non-operative management of patient with a CT scan demonstrated acute subdural hematoma may be proposed as follows; 1) GCS score greater than or equal to 13 since injury, 2) Absence of associated intraparenchymal hematoma, contusion or edema, 3) Midline shift of less than 10 mm, and 4) Absence of basal cisternal effacement23). There is no rule regarding what size a subdural hematoma must be to necessitate removal. Parenchymal post traumatic damage occurs frequently in patients with MHI, but the need for surgical intervention is rare7). In our study, approximately 14% of MHI patients showed hemorrhagic contusion. One of the most difficult decisions to make in treating traumatic intracerebral hematoma is whether to remove the clot or use conservative treatment. Guidelines for surgical treatment of traumatic intracerebral hematoma patients are as follows; 1) Neurologically deteriorating patients, 2) Hematoma with a volume greater than 20 mL in temporal, temporoparietal or cerebellum regions1), 3) Hematoma with a volume greater than 30mL in frontal or parieto-occipital regions, 4) Compression of mesencephalic cistern, midline shift of greater than 3 mm and subarachnoid hemorrhage8), and 5) Accompanying intracranial lesions such as EDH or SDH or depressed fracture.
Repeat CT scan must be planned in the case of non-operative treatment of the acute SDH and TICH. Repeat CT scans were conducted for patients with clinical deterioration or with new clinical symptoms. MHI patients with adverse outcomes, including death, were reported at 0.2-3.4% of all patients12,15,32). In our study, incidences of adverse outcome was 2% and death rate was 0.9%. With better treatment of MHI through improved risk-level assessment, we believe that deaths amongst MHI patients can be eliminated.
CONCLUSION
In our study, GCS score, LOC, patient age (> 65 years old) and skull fracture were identified as independent risk factors in patients presenting with intracranial lesions following MHI. Based on the risk levels for incidences of intracranial lesions, existing classification of MHI was further subdivided into four categories; very low, low, medium, and high risk groups. Treatment guidelines proposed for each category are as followings. CT scan is recommended for all head injury patients; patients with very low and low risk MHI may be discharged; medium and high risk MHI patients should be admitted for clinical observation because there are still a risk of deterioration; and high risk MHI patient should be treated as moderate head injury.
Acknowledgements
This work was supported by the Dongguk University Research Fund of 2009
References
- 1.af Geijerstam JL, Britton M. Mild head injury : reliability of early computed tomographic findings in triage for admission. Emerg Med J. 2005;22:103–107. doi: 10.1136/emj.2004.015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews BT, Chiles BW, 3rd, Olsen WL, Pitts LH. The effect of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg. 1988;69:518–522. doi: 10.3171/jns.1988.69.4.0518. [DOI] [PubMed] [Google Scholar]
- 3.Arienta C, Caroli M, Balbi S. Management of head-injured patients in the emergency department : a practical protocol. Surg Neurol. 1997;48:213–219. doi: 10.1016/s0090-3019(97)00019-0. [DOI] [PubMed] [Google Scholar]
- 4.Brown CV, Zada G, Salim A, Inaba K, Kasotakis G, Hadjizacharia P, et al. Indications for routine repeat head computed tomography (CT) stratified by severity of traumatic brain injury. J Trauma. 2007;62:1339–1344. doi: 10.1097/TA.0b013e318054e25a. discussion 1344-1345. [DOI] [PubMed] [Google Scholar]
- 5.Chen TY, Wong CW, Chang CN, Lui TN, Cheng WC, Tsai MD, et al. The expectant treatment of "asymptomatic" supratentorial epidural hematomas. Neurosurgery. 1993;32:176–179. doi: 10.1227/00006123-199302000-00004. discussion 179. [DOI] [PubMed] [Google Scholar]
- 6.Choi SW, Koh HS, Yeom JY, Kim SH, Song SH, Kim Y. Clinical analysis of the risk factors and prognostic factors of delayed deterioration following mild head injury. J Korean Neurosurg Soc. 1999;28:1316–1323. [Google Scholar]
- 7.Dacey RG, Jr, Alves WM, Rimel RW, Winn HR, Jane JA. Neurosurgical complications after apparently minor head injury. Assessment of risk in a series of 610 patients. J Neurosurg. 1986;65:203–210. doi: 10.3171/jns.1986.65.2.0203. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg HM, Gray HE, Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- 9.Gómez PA, Lobato RD, Ortega JM, De La Cruz J. Mild head injury : differences in prognosis among patients with a Glasgow Coma Scale score of 13 to 15 and analysis of factor associated with abnormal CT finding. Br J Neurosurg. 1996;10:453–460. doi: 10.1080/02688699647078. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton M, Wallace C. Nonoperative management of acute epidural hematoma diagnosed by CT : The neuroradiologist's role. AJNR Am J Neuroradiol. 1992;13:853–859. discussion 860-862. [PMC free article] [PubMed] [Google Scholar]
- 11.Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with mild head injury. J Engl J Med. 2000;343:100–105. doi: 10.1056/NEJM200007133430204. [DOI] [PubMed] [Google Scholar]
- 12.Hsiang JN, Yeung T, Yu AL, Poon WS. High-risk mild head injury. J Neurosurg. 1997;87:234–238. doi: 10.3171/jns.1997.87.2.0234. [DOI] [PubMed] [Google Scholar]
- 13.Ibañez J, Arikan F, Pedraza S, Sánchez E, Poca MA, Rodriguez D, et al. Reliability of clinical guidelines in the detection of patients at risk following mild head injury : results of a prospective study. J Neurosurg. 2004;100:825–834. doi: 10.3171/jns.2004.100.5.0825. [DOI] [PubMed] [Google Scholar]
- 14.Ingebrigtsen T, Romner B, Kock-Jensen C The Scandinavian Neurotrauma Committee. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries. J Trauma. 2000;48:760–766. doi: 10.1097/00005373-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Jeret JS, Mandell M, Anziska B, Lipitz M, Vilceus AP, Ware JA, et al. Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery. 1993;32:9–15. doi: 10.1227/00006123-199301000-00002. discussion 15-16. [DOI] [PubMed] [Google Scholar]
- 16.Jones JJ, Jeffreys RV. Relative risk of alternative admission policies for patients with head injuries. Lancet. 1981;2:850–853. doi: 10.1016/s0140-6736(81)91114-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim KH, Shin MS, Cho KH, Kim HK, Cho KG. Nonoperative management of acute epidural hematoma. J Korean Neurosurg Soc. 1988;17:709–716. [Google Scholar]
- 18.Klauber MR, Marshall LF, Luerssen TG, Frankowski R, Tabaddor K, Eisenberg HM. Determinants of head injury mortality : importance of the low risk patient. Neurosurgery. 1989;24:31–36. doi: 10.1227/00006123-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kwon JT, Park K, Kim YB, Min BK, Hwang SN, Suk JS, et al. Clinical course and outcome following mild head injury. J Korean Neurosurg Soc. 1992;21:1071–1079. [Google Scholar]
- 20.Livingston DH, Loder PA, Hunt CD. Minimal head injury : is admission necessary? Am Surg. 1991;57:14–17. [PubMed] [Google Scholar]
- 21.Marion DW, Carlier PM. Problems with initial Glasgow Coma Scale assessment caused by prehospital treatment of patients with head injuries : resulted of a national survey. J Trauma. 1994;36:89–95. doi: 10.1097/00005373-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Masters SJ, McClean PM, Arcarese JS, Brown RF, Campbell JA, Freed HA, et al. Skull x-ray examinations after head trauma. Recommendations by a multidisciplinary panel and validation study. N Engl J Med. 1987;316:84–91. doi: 10.1056/NEJM198701083160205. [DOI] [PubMed] [Google Scholar]
- 23.Mathew P, Oluoch-Olunya DL, Condon BG, Bullock R. Acute subdural hematoma in the conscious patient : outcome with initial non-operative management. Acta Neurochir (Wein) 1993;121:100–108. doi: 10.1007/BF01809258. [DOI] [PubMed] [Google Scholar]
- 24.Mendelow AD, Teasdale G, Jennett B, Bryden J, Hessett C, Murray G. Risks of intracranial hematoma in head injured adults. Br Med J (Clin Res Ed) 1983;287:1173–1176. doi: 10.1136/bmj.287.6400.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran SG, McCarthy MC, Uddin DE, Poelstra RJ. Predictors of positive CT scans in the trauma patient with minor head. Am Surg. 1994;60:533–535. discussion 535-536. [PubMed] [Google Scholar]
- 26.Murshid WR. Management of minor head injuries : admission criteria, radiological evaluation and treatment of complications. Acta Neurochir (Wien) 1998;140:56–64. doi: 10.1007/s007010050058. [DOI] [PubMed] [Google Scholar]
- 27.Nagurney JT, Borczuk P, Thomas SH. Elder patients with closed head trauma : a comparison with nonelder patients. Acad Emerg Med. 1998;5:678–684. doi: 10.1111/j.1553-2712.1998.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 28.Park YS, Kim HJ, Whang K, Pyen JS, Hu C, Hong SK, et al. Clinical characteristic and prognosis of mild head injury in the elderly. J Korean Neurosurg Soc. 2002;31:564–568. [Google Scholar]
- 29.Pozati E, Tognetti F. Spontaneous healing of acute extradural haematomas : Study of twenty-two cases. Neurosurgery. 1986;16:696–700. doi: 10.1227/00006123-198606000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Procaccio F, Stocchetti N, Citerio G, Berardino N, Beretta L, Della Corte F, et al. Guidelines for the treatment of adult with severe head trauma (part I). Initial assessment ; evaluation and pre-hospital treatment ; current criteria for hospital admission ; systemic and cerebral monitoring. J Neurosurg Sci. 2000;44:1–10. [PubMed] [Google Scholar]
- 31.Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- 32.Servadei F, Ciucci G, Loroni L, Cuscini M, Piola C, Arista A. Diagnosis and management of minor head injury : a regional multicenter approach in Italy. J Trauma. 1995;39:696–701. doi: 10.1097/00005373-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Servadei F, Ciucci G, Pagano F, Rebucci GG, Ariano M, Piazza G, et al. Skull fractures as a risk factors of intracranial complications in minor head injuries : a prospective CT study in series of 98 adult patients. J Neurol Neurosurg Psychiatry. 1988;51:526–528. doi: 10.1136/jnnp.51.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Servadei F, Faccani G, Roccella P, Seracchioli A, Godano U, Ghadirpour R, et al. Asymptomatic extradural haematomas. Results of multicenter study of 158 cases in minor head injury. Acta Neurochir (Wien) 1989;96:39–45. doi: 10.1007/BF01403493. [DOI] [PubMed] [Google Scholar]
- 35.Servadei F, Teasdale G, Merry G Neurotraumatology Committee of the World Federation of Neurosurgical Societies. Defining acute mild head injury in aults a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma. 2001;18:657–664. doi: 10.1089/089771501750357609. [DOI] [PubMed] [Google Scholar]
- 36.Stein SC, O'Malley KF, Ross SE. Is routine computed tomography scanning too expensive for mild head injury? Ann Emerg Med. 1991;20:1286–1289. doi: 10.1016/s0196-0644(05)81066-2. [DOI] [PubMed] [Google Scholar]
- 37.Stein SC, Ross SE. Moderate head injury : a guide to initial management. J Neurosurg. 1992;77:562–564. doi: 10.3171/jns.1992.77.4.0562. [DOI] [PubMed] [Google Scholar]
- 38.Stein SC, Ross SE. The value of computed tomographic scans in patients with low-risk head injuries. Neurosurgery. 1990;26:638–640. doi: 10.1097/00006123-199004000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Stein SC, Young GS, Talucci RC, Greenbaum BH, Ross SE. Delayed brain injury after head trauma : significance of coagulopathy. Neurosurgery. 1992;30:160–165. doi: 10.1227/00006123-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 41.Voss M, Knottenbelt JD, Peden MM. Patients who reattend after head injury : a high risk group. BMJ. 1995;311:1395–1398. doi: 10.1136/bmj.311.7017.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Yates D, Chater N, Cooper P, Dent H, Dunning J, Evans R, et al. Head injury. Triage, assessment, investigation and early management of head injury in infants, children, and adults. National Institute for Health and Clinical Excellence. 2007. [Accessed January 16, 2008]. For the National Collaborative Centre for Acute Care. Available at : http://www.nice.org.uk/nicemedia/pdf/CG56NICEGuideline.pdf.
- 44.Zimmerman RA, Bilaniuk LT. Computed tomographic staging of traumatic epidural bleeding. Radiology. 1982;144:809–812. doi: 10.1148/radiology.144.4.7111729. [DOI] [PubMed] [Google Scholar]