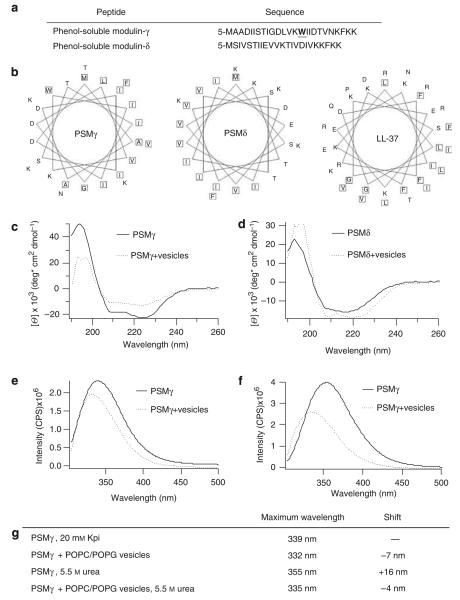
Figure 1. Phenol-soluble modulins have structural similarities and strongly interact with synthetic lipid membranes.
(a) Sequences of PSMγ and PSMδ, highlighting tryptophan in PSMγ. (b) Helical wheel plots show sequestration of hydrophobic residues for PSMγ, PSMδ, and LL-37. Circular dichroism spectra of 20 μm PSMδ (c) or PSMγ (d) in the presence and absence of 1 mm of 2:1 POPC/POPG lipid vesicles in 20 mm potassium phosphate buffer, pH 7.3, show an α-helical structure and structural changes of PSMδ and PSMγ in the presence of lipid vesicles. Tryptophan fluorescence spectra of PSMγ in the presence and absence of 1 mm of 2:1 POPC/POPG vesicles in 20 mm potassium phosphate buffer, pH 7.3 (e), or in the presence of 5.5 M urea (f). (g) Table displaying the maximum emission wavelength of PSMγ’s tryptophan. POPC/POPG vesicles in 20 mm of potassium phosphate buffer, pH 7.3 (Kpi), cause a blue shift in the tryptophan’s maximal emission indicating an embedment of PSMγ in the lipid membrane.