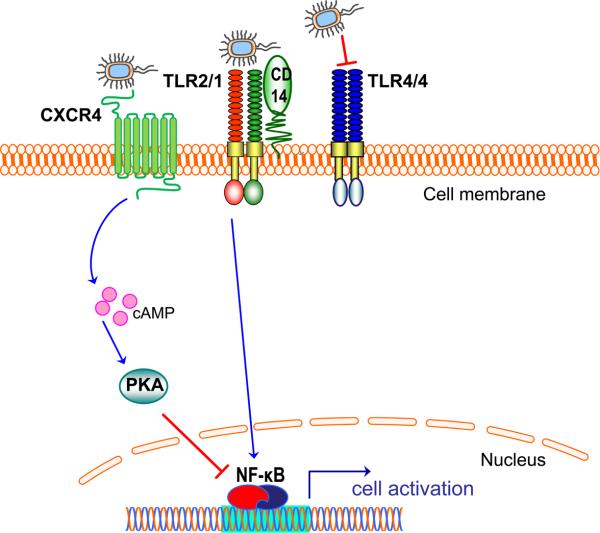
Fig. 3. Evasion or subversion of TLR activation by P. gingivalis.
P. gingivalis uses an elaborate system of lipid A phosphatase and deacylase activities that modify the lipid A structure of its lipopolysaccharide (21, 22). These modifications result in lipopolysaccharide molecules that can either evade or actively antagonize TLR4 activation (depicted as a homodimer; TLR4/4) (21, 22). Although the activation of the TLR2/TLR1 heterodimer (TLR2/1) is not antagonized at the TLR receptor level, P. gingivalis instigates a molecular cross-talk between the CXC-chemokine receptor 4 and TLR2/1. Unlike CD14 which facilitates TLR2/1 activation by the pathogen (57), CXCR4 suppresses TLR2 signaling (62). Mechanistically, P. gingivalis uses its fimbriae to bind CXCR4 and induce cAMP-dependent PKA signaling, which in turn inhibits the activation of nuclear factor-κB (NF-κB) activation (62).