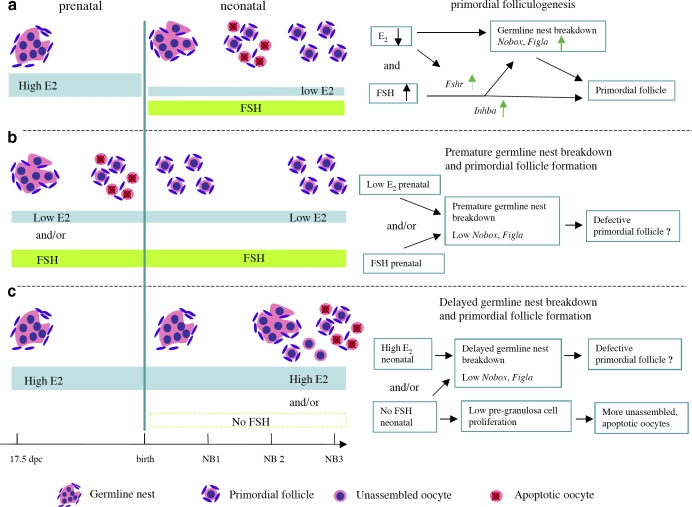
FIG. 7.
The role of FSH and E2 levels and timing in primordial folliculogenesis in in vitro-cultured mouse ovaries. a) During normal primordial follicle formation, represented in vitro by culturing 17.5-dpc mouse fetal ovaries for 3 days in high E2 and then 3 days in low E2 and FSH, a drop in E2 levels and presence of FSH permitted germline nest breakdown and up-regulation of oocyte expression of Figla and Nobox, which are critical for primordial folliculogenesis. At the same time, stimulation of Fshr gene expression and the activin (Inhba) signaling pathway conferred FSH responsiveness and promoted proliferation of pregranulosa cells. b) In the presence of persistently low E2 levels and/or with FSH addition, cultured fetal mouse ovaries exhibited premature germline nest breakdown and primordial follicle formation. Loss of the acute drop in E2 after birth (Day 4 of in vitro culture) leads to low levels of Figla and Nobox in oocytes, which might lead to the assembly of primordial follicles with defective oocytes. c) Exposure of cultured fetal ovaries to persistently high E2 levels inhibited germline nest breakdown, delayed primordial folliculogenesis, and repressed Figla and Nobox expression in oocytes. Loss of neonatal FSH exposure promoted oocyte apoptosis and decreased pregranulosa cell proliferation, which in turn led to incomplete primordial follicle formation.