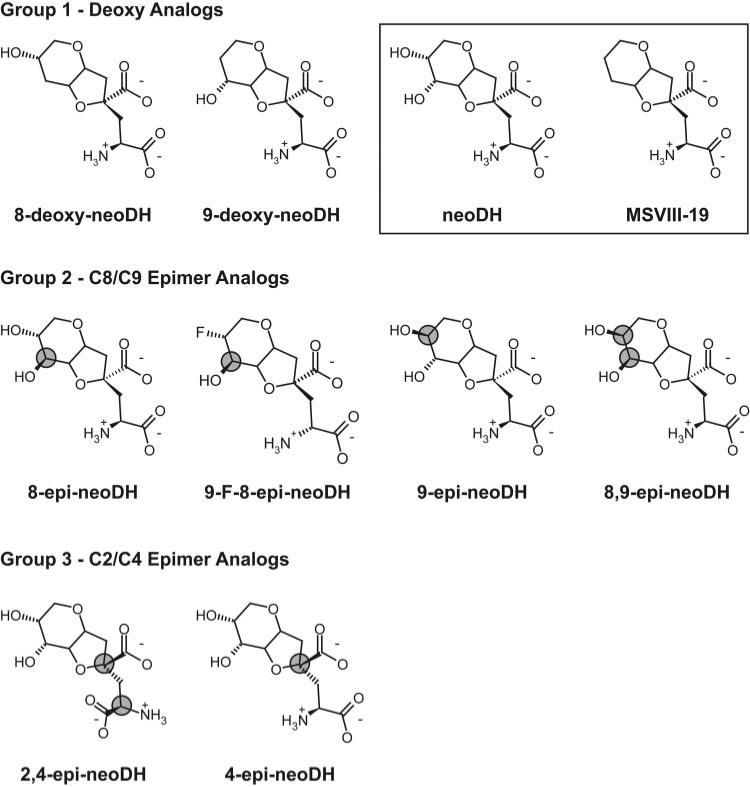
Fig. 1.
Chemical structures of the three groups of synthetic neodysiherbaine-derived analogs. The parent marine toxin neoDH and the first synthetic analog MSVIII-19 are outlined in a box in the first row of structures. neoDH contains a glutamate backbone connected to a rigid ring structure with hydroxyl groups at both the C8 and C9 ring positions; MSVIII-19 is the dideoxy synthetic analog. Group 1 analogs are the deoxy analogs at the C8 and C9 ring positions. Group 2 analogs are epimer analogs that manipulate the orientation of the substituents at the C8 and C9 ring positions. Group 3 analogs are epimer analogs at the C2/C4 positions within the glutamate backbone of the parent compound. The carbons changed are indicated by shaded gray circles.