Abstract
Background
Although an increasing body of evidence links being overweight in midlife with an increased risk for dementia in late life, no studies have examined the association between being overweight in midlife and cognitive ability in late life. Our aim was to examine the association between being overweight in midlife as measured by body mass index (BMI) and cognitive ability assessed over time.
Methods
Participants in the Swedish Adoption/Twin Study Aging were derived from a population-based sample. The participants completed baseline surveys in 1963 or 1973 (mean age 41.6 years, range 25–63 years). The surveys included questions about height, weight, diseases, and lifestyle factors. Beginning in 1986, the same individuals were assessed on neuropsychological tests every 3 years (except in 1995) until 2002. During the study period, 781 individuals who were 50 years and older (60% women) had at least one complete neuropsychological assessment. A composite score of general cognitive ability was derived from the cognitive test battery for each measurement occasion.
Results
Latent growth curve models adjusted for twinness showed that persons with higher midlife BMI scores had significantly lower general cognitive ability and significantly steeper longitudinal decline than their thinner counterparts. The association did not change substantially when persons who developed dementia during the study period were excluded from the analysis.
Conclusions
Higher midlife BMI scores precede lower general cognitive ability and steeper cognitive decline in both men and women. The association does not seem to be mediated by an increased risk for dementia.
Keywords: Body mass index, Cognition, Dementia, Epidemiology
THE adverse effects of being overweight are not limited to cardiac function but also extend to neurological function. A convincing body of evidence shows that being overweight in midlife is associated with an increased risk for dementia (1–5). However, to our knowledge, no previous study has assessed the association between body mass index (BMI) in midlife and changes in cognitive ability over an extended period of time. Currently, the only published study to assess the association between BMI and general cognitive ability over a long period is the Framingham Heart Study (FHS; 6,7), which showed that elderly obese men (mean age at baseline 65 years, range 55–88 years) had an increased risk for lower performance on a global composite score 18 years later as compared with nonobese men. However, this association was not observed among women. Other studies have shorter follow-up times (8) and a higher mean age at baseline (9–11).
The Swedish Adoption/Twin Study of Aging (SATSA) is ideal for studying the long-term association between BMI and cognitive function because (a) the participants have been followed for 40 years after baseline assessment of BMI; (b) cognitive ability has been measured five times over a 20-year period, making it possible to evaluate the rate of cognitive decline; (c) important covariates previously linked to BMI and cognitive functions, such as age, sex, lifestyle factors (education, smoking, and alcohol behaviors), diabetes mellitus, and cardiovascular diseases (CVDs), were assessed during the entire study period; and (d) dementia was screened for and diagnosed on an ongoing basis. We examined whether midlife BMI was associated with the level of cognitive performance in late life and examined the trajectories of cognitive ability as assessed by longitudinal models of cognitive change. Because the FHS found an association for men but not for women, we examined men and women separately.
METHODS
Participants
The study sample was drawn from the Swedish Twin Registry (12). The registry was compiled from two cohorts; the older cohort comprised same-sex twin pairs born before 1926. These participants were mailed questionnaires in 1963 and 1967 (or in 1970 for those who did not respond in 1967) that included questions about weight, height, smoking and alcohol habits, diseases and so forth. In 1973, a second cohort (born from 1926 through 1958) was mailed a questionnaire that included generally the same questions as had been asked of the older cohort.
In 1986, a subsample of the Swedish Twin Registry was invited to participate in the SATSA (13). The aim was to study gerontological genetics. The origin of the project dates to 1978 when it was observed that an appreciable number of twins in the Swedish Twin Registry had been reared apart from one another. All twins who had been reared apart and a sample of reared-together pairs matched for birth year, county of birth, and sex were invited to participate. The study design has been described in detail elsewhere (13). At the first in-person testing (IPT) in 1986, 645 twins 50 years of age and older participated. Since then, these twins and all twins who turned 50 years of age since the last IPT were systemically interviewed and assessed on a battery of neuropsychological tests every 3 years (except in 1995) by trained research nurses in a primary care facility close to their home. Among those who participated in the SATSA IPTs, the availability of BMI scores from midlife ranged from 89% to 61%, with higher availability for the first IPTs. The neuropsychological test battery included 11 tests (14). In total, 781 individuals (60% women) had both a midlife BMI score reported in the 1963 (56.5%) or 1973 (43.5%) mailed questionnaire and at least one completed neuropsychological assessment between 1986 and 2004.
BMI was calculated from self-reported data in 1963 and 1973 as weight in kilograms divided by the square of height in meters. Previous work with SATSA indicates a very high correlation of self-reported and measured height and weight (15). Level of education was dichotomized as low (≤6 years) or high (>6 years). Self-reported alcohol consumption, smoking, and CVDs were evaluated in midlife using the 1967 or 1973 surveys, as well in the SATSA IPTs. Participants who reported that they never used alcohol during the entire study period were coded as nondrinkers; participants who reported that they had smoked at any time were coded as smokers. Self-reported data on heart attack, angina pectoris, heart insufficiency, diabetes, and stroke a least once during the study period were coded as presence or absence. Persons who reported use of antihypertensive medication and/or an assessed blood pressure above 140/90 mmHg twice or more during the IPTs were coded as hypertensive. The diseases were summed to form CVD scores, which ranged from 0 to 7, with one point given if the disease or symptom was ever reported.
To create a measure of general cognitive ability, individual scores on the first principal component of all cognitive measures were obtained at IPT 1 (14). To avoid adding any error to the latent growth curve model by using a principal component that varied in definition at each time point, we standardized scores on each cognitive measure using the means and variance observed at IPT 1, creating an identical metric for each cognitive measure at all five time points. Next, we created a global cognitive factor for each testing occasion by combining the now-standardized cognitive scores using the factor loadings from the principal component analysis conducted at IPT 1, thereby ensuring that the definition of the cognitive factor remained constant across testing occasions. Lastly, the factor scores were scaled into t score metrics (16,17).
Dementia was continuously screened for during the IPTs. The selection criteria were as follows: participants with low scores on the Mini-Mental State Examination and cognitive tests, with a history of dementia in their medical records, with suspicion of dementia by the research nurses, who scored poorly on a telephone interview, and/or with information from refuser protocols (ie, a proxy reported that the twin had cognitive problems (18)). All suspected cases of dementia were diagnosed during a consensus conference according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R and DSM-IV, 4th edition) criteria (19). All available information (research protocols, medical records, and nurses’ notes) was used. During the study period, 68 participants were diagnosed with dementia.
Statistical Analysis
Differences between the study groups were assessed by chi-square test or t test, as appropriate, using SPSS version 15.0 (20).
In this study, we employed latent growth curve modeling to measure change in cognitive performance over time and to explore the potential effect of BMI on cognitive performance over time. Latent growth curve models measure and allow for comparisons of individual trajectories of decline as well as an average trajectory of decline across the entire sample. Individual changes are assumed to follow the mean path of change for the total population, but the random effects allow the individual levels of function to be higher or lower and the rate of decline or growth to be faster or slower.
Phenotypic latent growth model with a full maximum likelihood estimate technique was used in the growth models (21,22). Both linear and quadratic models were considered. Because we could not assume that the twins were independent of each other, models were adjusted to account for the correlation within twin pairs. PROC MIXED (23) was used to fit the latent growth curve models.
Growth curves were fit to establish linear and nonlinear age trends for general cognitive ability at the mean-centered age of 65 (linear) and 65 squared (nonlinear), the age at which cognitive abilities are considered to begin to decline (17). BMI was centered at 25, the value considered to be the breaking point between normal weight and overweight (24).
A stepwise procedure was adopted to evaluate longitudinal trajectories. Interaction terms between linear and quadratic age, sex, and BMI scores were added to the model. We used −2 log-likelihood test to evaluate the multiparameter hypothesis testing, starting with the full model that included all interaction terms and covariates, followed by stepwise exclusion of interaction terms and covariates that did not add anything to the model.
At each step, we controlled for age, educational level, alcohol use, smoking, and CVDs. Moreover, cohort was controlled for because the members of the younger cohort had a shorter follow-up time from baseline. Because SATSA data included individuals with dementia, all analyses were carried out twice: once including all persons with dementia and once excluding them.
RESULTS
Study Sample Characteristics
The mean age at midlife was 41.6 years (range 25–63 years). Participant characteristics according to sex are presented in Table 1. At midlife, the mean BMI was 23.7 (range 17.1–38.5); 2% of the sample were underweight (BMI < 18.5), 68.4% normal weight (18.5 – 24.9), 25.9% overweight (25 – 29.9), and 3.7% obese (BMI ≥ 30). The composite score of general cognitive ability ranged from 22.81 to 75.73 during the study period, with higher scores indicating better cognitive performance. In general, men had a nonsignificantly higher level of cognitive functioning than women, as illustrated in Table 1 by values from IPT 1. There was no significant difference in mean BMI between the 68 participants who developed dementia and those who were cognitively intact at death or in 2005.
Table 1.
Sample Characteristics by Sex (N = 781)
| Men, n = 307 | Women, n = 474 | p Value | |
| Age in 1986, mean (SD) | 59.6 (10.2) | 61.6 (11.2) | .007 |
| Midlife BMI, mean (SD) | 24.2 (2.6) | 23.3 (3.3) | .000 |
| CVDs*, mean (SD) | 2.0 (0.1) | 1.9 (0.1) | .288 |
| Low education (≤6 y), n (%) | 131 (47.1) | 147 (52.9) | .281 |
| Alcohol abstainers, n (%) | 23 (7.1) | 113 (23.3) | .000 |
| Present/ex-smoker, n (%) | 245 (75.9) | 207 (42.6) | .000 |
| Cognitive ability† at IPT 1 (n = 574), mean (SD) | 50.9 (10.7) | 49.4 (9.7) | .085 |
Notes: BMI = body mass index; IPT = in-person testing.
CVDs, cardiovascular diseases—including self-reported heart attack, angina pectoris, heart insufficiency, high blood pressure, thrombosis, stroke, and diabetes during the study period.
First principal component.
Midlife BMI and Cognitive Functioning
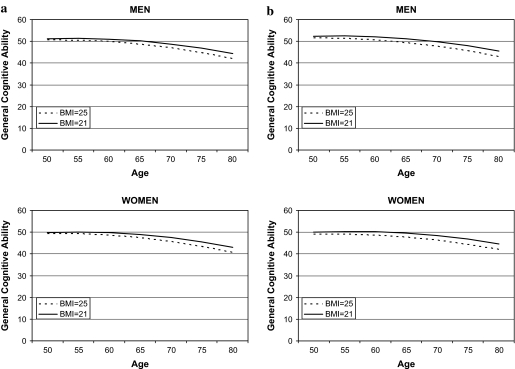
Significant average performance effects (intercept) on general cognitive ability were found for BMI in midlife when educational level, cohort, alcohol use, smoking, and CVDs were controlled for (Table 2). Persons with a higher BMI in midlife had lower general cognitive ability in late life. Moreover, their cognitive ability declined faster, as indicated by the interaction term between age and BMI (Table 2). The trajectory of change in global cognitive function from mid- to late life by BMI (21 and 25) is illustrated in Figure 1a. There were no significant interaction terms between BMI, sex, linear age, and quadratic age, and adding these interaction terms did not significantly improve the model, as evaluated by the –2 log-likelihood test.
Table 2.
Relationship Between Midlife Body Mass Index (BMI) and Changes in General Cognitive Ability, Controlling for Cohort, Education, Cardiovascular Diseases, Smoking, and Alcohol Use
| Model Term | All |
Excluding Persons With Dementia |
||
| Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Age* | −0.288 (0.020) | <.0001 | −0.285 (0.020) | <.0001 |
| Age2† | −0.011 (0.001) | <.0001 | −0.010 (0.001) | <.0001 |
| BMI‡ | −0.348 (0.112) | .002 | −0.434 (0.118) | .0002 |
| Age × BMI§ | −0.015 (0.006) | .011 | −0.015 (0.006) | .012 |
Notes: *Linear longitudinal change defined by age.
Curvilinear longitudinal change defined by age.
Intercept, difference in mean BMI scores.
Slope, difference in trajectory of change due to age and BMI scores.
Figure 1.
(a) Longitudinal association between midlife body mass index (BMI) scores and general cognitive ability measured by the first principal component, including persons with dementia. (b) Longitudinal association between midlife BMI scores and general cognitive ability measured by the first principal component, excluding persons with dementia.
Women had a nonsignificant lower general cognitive ability than men (β −1.635, p = .064). However, there were no significant interaction terms between sex and linear age, quadratic age, or BMI, demonstrating that there were no sex differences in the intercept or the slope, as illustrated in Figure 1a. When persons diagnosed with dementia during the study period were excluded from the analysis, the association between midlife BMI and cognitive function became somewhat stronger (Table 2). The trajectory remained about the same (Figure 1b).
DISCUSSION
The results show that a higher BMI, representing being overweight, was associated with lower general cognitive ability and a more rapid decline in cognitive ability among both men and women. When persons who developed dementia over the course of the study were excluded, the pattern remained about the same.
Few comparable studies have evaluated the association between midlife BMI and general cognitive ability in late life. The FHS reported that elderly obese men had an increased risk for a lower level of general cognitive ability 18 years later (6,7), although they did not find the same association in women. In this study, we did find an association between higher midlife BMI and lower general cognitive ability in later life for both men and women. The most important difference between the two studies is the baseline age at which BMI was assessed. We measured BMI in middle age (mean age 44 years), whereas the FHS assessed BMIs among young elderly patients (mean age 66 years). The time point of BMI assessment seems to be of importance; several studies have reported that midlife high BMI is associated with a higher risk for dementia (1–4), whereas in late life, high BMI or weight is in most studies associated with an decreased risk for dementia (10,25–29).
Our study showed that the association between midlife BMI and general cognitive ability remained even when persons diagnosed with dementia at any time during the study period were excluded from the analysis. Hence, adiposity might influence cognitive functioning independently from dementia. Despite the extensive evaluation of dementia in the present study, the possibility that those individuals who experienced more rapid cognitive decline were in preclinical stages of dementia cannot be ruled out as previous studies have shown that cognitive function starts to decline more than 10 years before the clinical onset of dementia (30–32).
There are several pathways by which being overweight might increase the risk for lower cognitive function. Being overweight is a common denominator in CVD, and being overweight is also related to inflammation. Both CVD and inflammation have been linked to an increased risk for cognitive impairment and dementia (33). Still, most studies, including ours, show an association between being overweight and cognitive impairment even when CVD and diabetes are controlled for. However, this does not rule out the possibility that CVD is the link between overweight and cognitive decline, particularly when considering that both hypertension- and diabetes-related conditions are underdiagnosed in the general population (34) and hence not captured and controlled for.
The main strengths of our study include its population-based design, the long follow-up time with repeated evaluations of cognitive function using a battery of cognitive tests, and a midlife perspective of CVD and lifestyle factors. A composite cognitive outcome measure has several advantages over single measures of cognitive function; for example, composite measures reduce the sources of measurement error such as different difficulty levels and floor and ceiling effects (35). In our study, dementia was diagnosed using an extensive research protocol that included several cognitive tests and nurses’ evaluations, which have previously been shown to capture most dementia cases in a Swedish setting (36). Nevertheless, there are limitations that need to be discussed. Although BMI is correlated with fat mass (37,38), it does not assess body fat distribution, which has been linked to the development of CVD. For example, it is a well-known fact that waist circumference is a stronger predictor of CVD diseases than BMI. Accordingly, Whitmer and colleagues (4) reported that central obesity is a better predictor of dementia than BMI. Unfortunately, neither waist circumferences nor any other anthropometric measures were available in our study at baseline. It should also be mentioned that the proportions of participants in this study who were overweight or obese were relatively low, which might be due to the fact that height and weight were self-reported. Nevertheless, previous analyses of SATSA demonstrate that there is a very high correspondence between measured and self-reported weight (15). Furthermore, the proportions from this study were representative of the proportions of overweight and obese persons in the Swedish population in 1960 and 1970.
Implication
These findings suggest that reducing the BMIs of individuals in midlife might represent a strategy for improving cognitive function in late life.
FUNDING
Data collection was supported by the National Institute on Aging (AG04563, AG10175, AG08724), the MacArthur Foundation Research Network on Successful Aging, and the Swedish Council for Social Research (97:0147:1B). Analytic work was supported by a grant from the Bank of Sweden Tercentenary Foundation.
References
- 1.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry C, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Br Med J. 2005;330:1360–1364. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalmijn S, Foley L, White CM, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: the Honolulu-Asia Aging Study. Arterios Thromb Vasc Bio. 2000;20:2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 5.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 7.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol Aging. 2005;26:11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadra S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 9.Brubacher D, Monsch A, Stähelin H. Weight change and cognitive performance. Int J Obes. 2004;28:1163–1167. doi: 10.1038/sj.ijo.0802721. [DOI] [PubMed] [Google Scholar]
- 10.Buchman A, Wilson R, Bienias J, Shah R, Evans D, Bennett D. Change in body mass index and risk of incident Alzheimer's disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 11.Sturman MT, Mendes de Leon CF, Bienias JL, Morris JC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein P, deFaire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 13.Finkel D, Pedersen N. Processing speed and longitudinal trajectories of change for cognitive abilities: the Swedish adoption/twin study of aging. Aging Neuropsychol Cogn. 2004;11:325–345. [Google Scholar]
- 14.Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychol Sci. 1992;3:346–352. [Google Scholar]
- 15.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 16.Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- 17.Finkel D, Reynolds C, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39:535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- 18.Gatz M, Pedersen N, Berg S, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 20.SPSS. SPSS Advanced Models Version 15.0. Chicago, IL: SPSS; 2007. [Google Scholar]
- 21.McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol. 2002;38:115–142. [PubMed] [Google Scholar]
- 22.McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary psychological research. In: Schinka J, Velicer W, editors. Comprehensive Handbook of Psychology. Vol. 3. New York, NY: Wiley; 2003. pp. 447–480. [Google Scholar]
- 23.SAS Institute Inc. SAS System for Microsoft Windows Version 9.1. SAS Institute Inc., Cary, NC; 2002–2003. [Google Scholar]
- 24.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. World Health Organization:: Geneva, Switzerland; 1995. [Google Scholar]
- 25.Luchsinger JA, Patel B, Tang M-X, Schupf N, Mayeux R. Measures of adiposity and dementia risk in the elderly. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nourhashémi F, Deschamps V, Larrieu S, Letenneur L, Dartigues J-F, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 27.Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia. Arch Neurol. 2005;65:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Cronin-Stubbs D, Beckett LA, Scherr PA, et al. Weight loss in people with Alzheimer's disease: a prospective population based analysis. BMJ. 1997;314:178–179. doi: 10.1136/bmj.314.7075.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen project. J Am Geriatr Soc. 2008;56:111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 30.Linn R, Wolf PA, Bachman DL. The “preclinical phase” of probable Alzheimer's disease: a 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 31.Elias MF, Beiser A, Wolf PA, Au R, White R, D'Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 32.La Rue A, Jarvik L. Cognitive function and prediction of dementia in old age. Int J Aging Hum Dev. 1987;25:79–89. doi: 10.2190/DV3R-PBJQ-E0FT-7W2B. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 34.Williams D, Wareham N, Brown D, et al. Undiagnosed glucose intolerance in the community: the Isle of Ely Diabetes Project. Diabet Med. 1994;12:30–35. doi: 10.1111/j.1464-5491.1995.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 36.Dahl A, Berg S, Nilsson S. Dementia identification in epidemiological research: a study on the usefulness of different data sources. Aging Clin Exp Res. 2007;19:381–389. doi: 10.1007/BF03324718. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 38.Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]