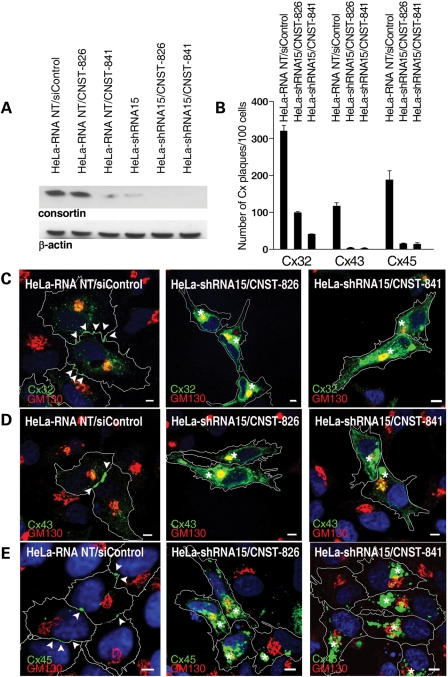
Figure 7.
Consortin silencing alters the plasma membrane targeting of connexins 32, 43 and 45. (A) Immunoblot of consortin in lysates of HeLa-RNA-NT (stably expressing a non-silencing control shRNA) and HeLa-shRNA15 cells (stably expressing a consortin-targeting shRNA), after 72 h of siRNA-mediated consortin knockdown. Ten microgram total cellular protein extract were loaded per lane and β-actin was used as loading control. Maximum consortin silencing was obtained when combining shRNA stable knockdown with transfection of either CNST-826 or CNST-841 siRNAs. (B) Effect of consorting silencing on the number of connexin immunolabeled plaques in connexin-expressing HeLa-RNA-NT and HeLa-shRNA15 cells after 72 h of siRNA-mediated consortin knockdown. In all cases, differences between control and consortin-silenced cells were statistically significant (Supplementary Material, Table S1). (C–E) Effects of consortin knockdown on connexin distribution in HeLa/RNA-NT and HeLa-shRNA15 cells expressing Cx32 (C), Cx43 (D) or Cx45-eGFP (E) after 72 h of siRNA transfection. Cells were stained for the appropriate connexin (green) and the Golgi marker GM130 (red), and counterstained with DAPI (blue). Cell contours are drawn as white lines. Arrowheads indicate immunostained connexin plaques at intercellular contacts. Note the absence of plaques and the intracellular connexin accumulation (asterisks) in cells undergoing consortin silencing. Bars, 5 µm.