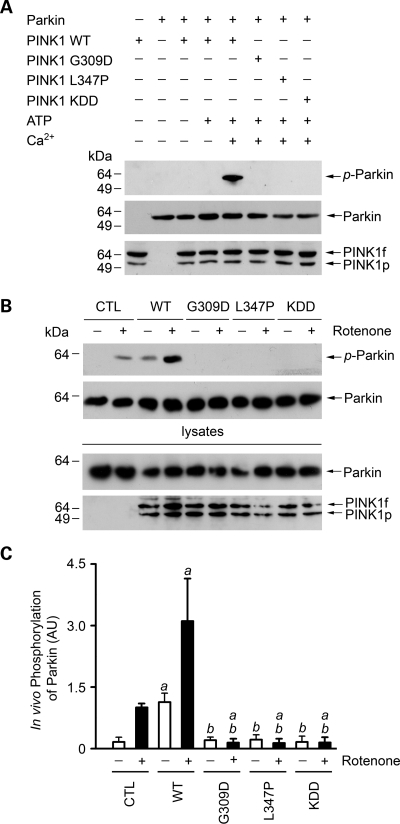
Figure 3.
Phosphorylation of parkin by wild-type and mutant PINK1. (A) In vitro phosphorylation assays were performed by incubation of purified Myc-tagged parkin and FLAG-tagged wild-type (WT) or mutant PINK1 proteins as indicated in the absence or presence of 0.1 mm ATP and 0.5 mm CaCl2. Phosphorylation of parkin was detected by immunoblotting with anti-phosphoserine antibody (top panel), and the amounts of PINK1 and parkin proteins used in the phosphorylation assays were shown by immunoblotting with anti-Myc (middle panel) and anti-FLAG antibodies (bottom panel). (B) SH-SY5Y cells expressing Myc-tagged parkin and wild-type or mutant PINK1 or the vector-transfected control (CTL) were treated with 20 nm rotenone for 8 h as indicated. In vivo parkin phosphorylation was determined by immunoprecipitated with anti-Myc antibody followed by immunoblotting using anti-phosphoserine and anti-parkin antibodies. (C) Normalized levels of in vivo parkin phosphorylation by PINK1. Data represent mean ± SEM from three independent experiments. aSignificantly different from the corresponding vector-treated controls (P < 0.05). bSignificantly different from the corresponding wild-type PINK1-transfected controls (P < 0.05). AU, arbitrary units.