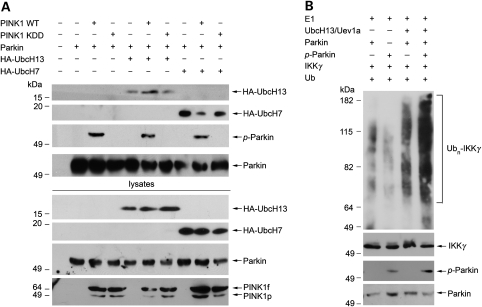
Figure 5.
Phosphorylation of parkin by PINK1 enhances the ability of parkin to bind UbcH13 and catalyze K63-linked polyubiquitination. (A) Lysates from SH-SY5Y cells expressing Myc-tagged parkin, HA-tagged UbcH7 or UbcH13 and wild-type or mutant PINK1 constructs were immunoprecipitated with anti-Myc antibody followed by immunoblotting with anti-HA, anti-phosphoserine and anti-parkin antibodies. (B) In vitro ubiquitination assays were performed by incubation of purified FLAG-tagged IKKγ, non-phosphorylated parkin or PINK1-phosphorylated parkin in the presence of E1, E2 (UbcH13/Uev1a) and ubiquitin as indicated. Ubiquitination of IKKγ was detected by immunoprecipitation with anti-FLAG antibody followed by immunoblotting using anti-ubiquitin antibody. The amounts of IKKγ, non-phosphorylated parkin and PINK1-phosphorylated parkin used in the ubiquitination assays (Input) were shown by immunoblotting with anti-FLAG, anti-parkin and anti-phosphoserine antibodies. Ubn-IKKγ, polyubiquitinated IKKγ.