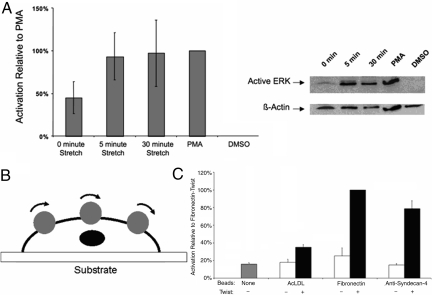
Fig. 4.
Activation of ERK under mechanical stimulation through syndecan-4. (A) Western blot and densitometry analysis for mechanical strain that was applied to the NIH 3T3 cells attached through anti-syndecan-4 antibodies for 0, 5, and 30 min. PMA-treated cells are a positive control for ERK phosphorylations, and the resulting level of relative activation was defined as 100% for comparison between experimental trials. DMSO-treated cells were a negative control for chemical activation of ERK phosphorylation. The results for a 0-min strain were significantly different from the 5-min strain (P < 0.025) and the 30-min strain (P < 0.025) based on t tests of log10-transformed data with a Bonforroni correction (α = 0.05/2) to treat for repeated testing. Each bar represents the average of at least three independent experiments. (B and C) Using an OMTC device (B) we obtained ERK activation measurements (C) with ELISA-based analysis of lysates of NIH 3T3 cells subjected to strain by twisting coated beads at 1 Hz for 30 min. The resulting ELISA data were normalized based on lysate protein concentration, and then the resulting values were scaled based on defining the twist treatment of fibronectin beads as 100% activation. The resulting ELISA T tests of selected comparisons among the resulting data show that the twist treatment of anti-syndecan-4-coated beads was significantly different with the no-bead sample (P < 0.0167), the no-twist anti-syndecan-4-coated bead sample (P < 0.0167), and the twist AcLDL-coated bead sample (P < 0.0167), using a Bonferroni correction (α = 0.05/3) to treat for repeated testing. Each bar represents the average of three independent experiments.