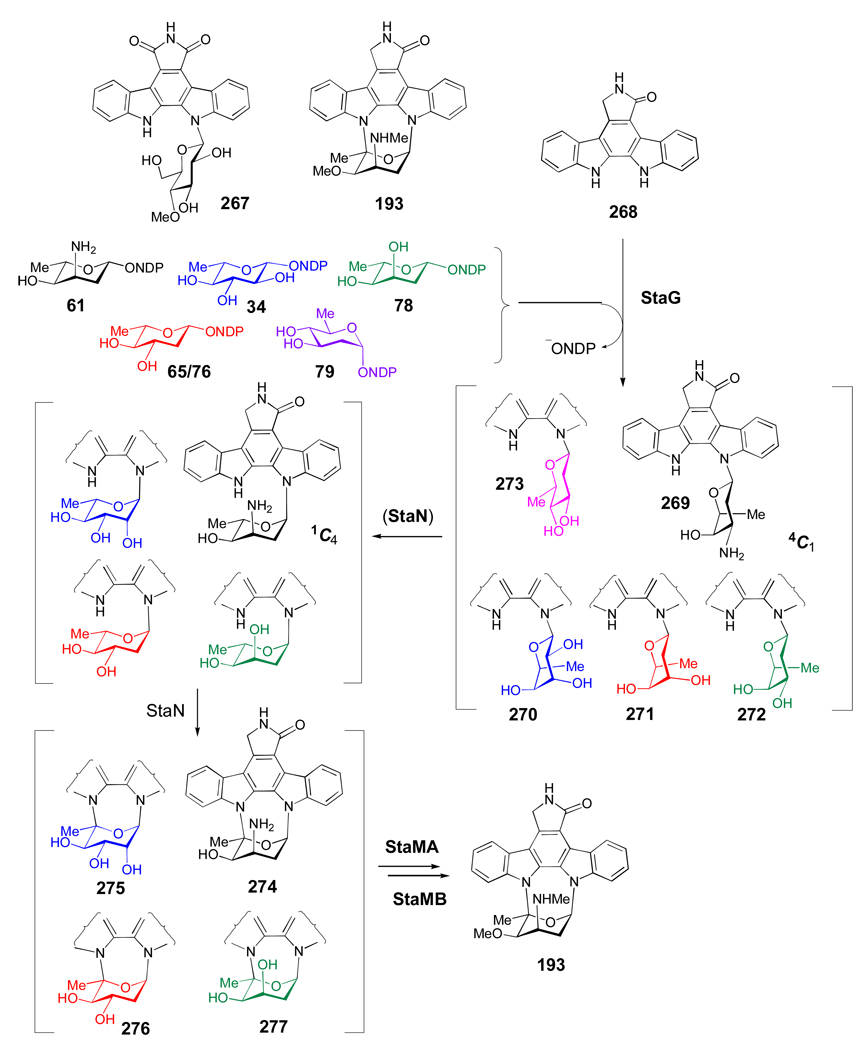
Figure 11. Novel indolocarbazoles generated by combinatorial biosynthesis in Streptomyces albus.
The indolocarbazoles rebeccamycin (267) and staurosporine (193) both contain unusual N-glycosidic linkages. Staurosporine biosynthesis was reconstituted in Streptomyces albus by expressing genes required for the formation of the staurosporine aglycone (268), genes encoding production of different deoxysugars (34, 61, 65, 78, and 79), along with the N-GT (StaG) and the P450 enzyme (StaN) responsible for the oxidative crosslinking between C5′ of the sugar and the N-12 atom of the aglycone. While StaG coupled both l- and d-sugars to 268, only the l-sugars could be oxidatively crosslinked by StaN to give 193 and 274–277.