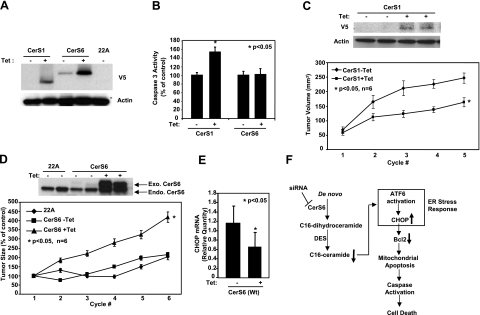
Figure 7.
Roles of CerS1 and CerS6 in the regulation of HNSCC tumor growth. A) UM-SCC-22A cells expressing C-terminal V5-tagged CerS1 or CerS6 under a Tet-inducible promoter were either noninduced (−Tet) or induced with Tet (1 μg/ml; +Tet) for 48 h, and CerS1 and CerS6 protein expression was detected by Western blotting using V5 antibody. Untransfected UM-SCC-22A cells (22A) were used as controls. B) Activation of caspase 3 was measured without or with Tet induction (48 h) in cells expressing CerS1 or CerS6. Bars represent means ± sd from 3 experiments. C–E) HNSCC xenografts were generated on the flanks of SCID mice using control UM-SCC-22A (22A) cells and UM-SCC-22A cells expressing CerS1 or CerS6 under a Tet-inducible promoter, as described in Materials and Methods. Sizes of the xenografts were measured every 3 d with calipers. Mice with the described xenografts were sacrificed, and CerS1 and actin protein (C), CerS6 (D), and CHOP (E) levels of the xenografts were detected by Western blotting and by Q-PCR. Bars represent means ± sd. *P < 0.05. F) Summary of data presented in this study. Results revealed that knockdown of CerS6 using siRNA initiates a specific arm of the ER stress response through the ATF6/CHOP pathway, which then leads to induction of caspase-dependent apoptosis.