Figure 1.
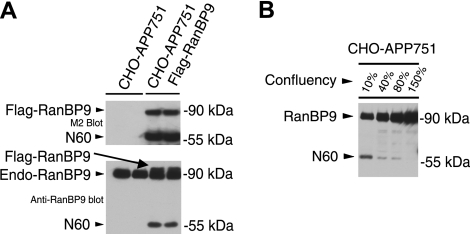
RanBP9 is proteolytically cleaved to generate RanBP9-N60 in CHO cells. A) CHO-APP751 parental cells and CHO-APP751-flag-RanBP9 stable cells were plated in duplicates, and confluent cultures were subjected to cell lysis and immunoblotting (equal protein amounts). Anti-Flag M2 antibody detected Flag-RanBP9 from stable cells but not from CHO-APP751 control cells (top panel, bottom band), demonstrating that stable cells express transfected RanBP9. In addition to the 90-kDa band expected of full-length RanBP9, an apparent proteolytic N-terminal 60-kDa fragment (RanBP9-N60) was also strongly detected with M2 antibody (top panel, bottom band). When lysates were probed with anti-RanBP9 antibody, endogenous hamster RanBP9 could be detected together with slightly slower migrating transfected human Flag-RanBP9 (bottom panel, arrow). N-terminal 60-kDa fragment was also detected with RanBP9 antibody, which was preferentially increased in RanBP9-transfected cells (bottom panel, bottom band). B) Regulation of RanBP9-N60 formation by cell density and microtubule dynamics. CHO-APP751 cells were plated at indicated densities and lysed in equal volumes, and equal volumes of lysates were probed with anti-RanBP9 monoclonal antibody. Note that N60 is formed only at low density and its formation is gradually reduced as cell density is increased, despite huge excess in protein in higher-density cultures.