Abstract
P-glycoprotein (Pgp) is an ATP-dependent efflux pump involved in transport of xenobiotics from cells that, when overexpressed, can mediate multidrug resistance in mammalian cells. Pgp may be a candidate target for new anthelmintics, as it plays critical roles in normal cell physiology, in removal of drugs from cells, and potentially in the development of drug resistance. Schistosomes are parasitic flatworms that cause schistosomiasis, which affects hundreds of millions of people worldwide. Here, we express SMDR2, a Pgp homologue from Schistosoma mansoni (Platyhelminthes), in Chinese hamster ovary (CHO) cells and use fluorescence-based assays to examine the functional and pharmacological properties of this transporter. Membrane vesicles from stably transfected CHO cells expressing recombinant SMDR2 show significant increases in rhodamine transport and ATP hydrolysis compared with those from control cells or cells transfected with empty vector. SMDR2-mediated transport is inhibited by the Pgp modulators verapamil (IC50=12.1 μM) and nifedipine, and also by praziquantel, the current drug of choice against schisotosomiasis (IC50=17.4 μM). Efflux measurements of a fluorescent analog of praziquantel indicate that it is also a substrate for SMDR2. The interaction of praziquantel with SMDR2 may offer new strategies for potentiating the action of praziquantel and possibly overcoming drug resistance.—Kasinathan, R. S., Goronga, T., Messerli, S. M., Webb, T. R., Greenberg, R. M. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel.
Keywords: verapamil, drug resistance, efflux transporter
Schistosomiasis affects an estimated 200 million people worldwide, ∼85% of whom live in Africa (1,2,3). The causative agents of schistosomiasis are trematode flatworms of the genus Schistosoma, which reside in the blood vessels of the host, take up nutrients from blood, and, presumably aided by cellular efflux pumps, excrete toxic metabolites and xenobiotics (4). These transporters can also transport drugs out of cells and are associated with development of drug resistance. Here, we determine the pharmacological properties of SMDR2, one of these efflux transporters from Schistosoma mansoni, showing that the antischistosomal drug praziquantel (PZQ) inhibits SMDR2-mediated uptake of rhodamine and is also a substrate for SMDR2.
PZQ is the current drug of choice against schistosomiasis and is effective against all schistosome species (5,6,7,8). However, the mode of action of PZQ has not been defined with certainty. Most evidence points to a role for a disruption of Ca2+ homeostasis within the worm (9,10,11), likely via increased flux through voltage-gated Ca2+ (Cav) channels (7). Other mechanisms and molecular targets have also been proposed and investigated (12,13,14,15), and the significance of Ca2+ influx in PZQ action has recently been questioned (16). These uncertainties regarding PZQ mode of action, as well as the withdrawal from the market of other commercially available antischistosomals, render the prospect of emerging PZQ resistance of particular concern (8, 17). It is therefore critically important to identify new antischistosomal drug targets, as well as compounds that might potentiate the effectiveness of current anthelmintics.
ATP-binding-cassette (ABC) transporters are involved in the export of biomolecules from cells (reviewed in ref. 18). One member of this class, P-glycoprotein (Pgp), is an ATP-dependent transporter that mediates efflux of toxic and xenobiotic compounds (reviewed in refs. 19, 20). The phenomenon of multidrug resistance (MDR) was initially noted in mammalian tumor cells that had been selected for resistance to a single drug but that also showed an unpredictable cross-resistance against several structurally unrelated compounds (21). The MDR phenotype is linked to amplification and overexpression of Pgp in certain mammalian tumor cells that show broad drug resistance (19, 20, 22, 23). Similarly, the Plasmodium falciparum MDR gene (pfmdr1) has been implicated in altering parasite susceptibility to a variety of currently available antimalarial drugs (24). Recently, the modulation of MDR transporters has been proposed as a potential strategy to enhance anthelmintic efficacy (25). Furthermore, expression levels and allele frequencies of Pgp may play a role in anthelmintic-resistant populations of nematodes (26,27,28,29,30,31). However, other than our recent study (32), we are aware of no evidence for an association of MDR transporters with drug resistance in schistosomes or other parasitic platyhelminths.
The SMDR2 gene encodes a S. mansoni Pgp-like protein, with 12 predicted transmembrane regions and two ATP-binding domains (33), but investigation of the functional properties of this and other schistosome efflux transporters has been limited. Sato et al. (34, 35) have used fluorescent substrates of Pgp and MDR-like proteins to visualize the excretory system of S. mansoni, and, subsequently, PZQ was shown to have significant effects on worm excretion of resorufin, a likely Pgp substrate (refs. 36, 37; reviewed in ref. 4). Interestingly, PZQ inhibits human Pgp-mediated [3H]taxol transport in a mammalian intestinal epithelial cell line, with a Kiapp of 20 μM (38).
In this study, we use heterologous expression and a variety of assays to show that SMDR2 is a functional ATP-dependent efflux transporter. Furthermore, we show that SMDR2 activity is potently inhibited by PZQ, as well as the classic Pgp inhibitors (and Cav channel blockers) verapamil and nifedipine. We also show that PZQ is a substrate for SMDR2. These results are discussed in reference to further understanding PZQ action and metabolism, as well as in regards to the reported effects of drugs such as verapamil and nifedipine on egg laying in schistotosomes and other flatworms (39).
MATERIALS AND METHODS
Cell culture and reagents
Chinese hamster ovary (CHO)-K1 cells from American Type Culture Collection (Manassas, VA, USA) were maintained in DMEM/Ham’s F-12 medium supplemented with 5 mM glutamine, 10% FBS, and 1% penicillin/streptomycin. Stable CHO cell lines expressing SMDR2 or empty vector were selected with 300 μg/ml of G418 sulfate (Cellgro, Manassas, VA, USA) and maintained in the complete medium plus 150 μg/ml of G418. Where mentioned, calcein-AM, sodium azide, ouabain, sodium-orthovanadate, verapamil, and PZQ (all from Sigma, St. Louis, MO, USA) and nifedipine (Spectrum, Gardena, CA, USA) were included in the assay buffer or pretreated with cells or membrane vesicles.
Cloning of SMDR2 cDNA
To obtain the full-length coding region of SMDR2 (accession no. L26287), total RNA from adult worms was isolated using RNAqueous-4-PCR (Ambion, Austin, TX, USA) and subjected to RT-PCR. First-strand synthesis was primed with 0.5 μg random hexamer/μg RNA and reverse transcribed at 42° for 2 h with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Primers designed against the 5′ end (SMDR2-3: GGGAATTGCCACCATGGCTAAGACACAGTCATCCAAT) and 3′ end (SMDR2-4: GGGCGGCCGCCTCATCATTATTTTGTATTATCTAATTA) of the coding region and containing restriction sites (5′-EcoR I, NcoI; 3′-NotI) were used to amplify the full-length coding sequence using the high-fidelity enzyme mix LA-Taq (Takara, Shiga, Japan) according to the manufacturer’s instructions, with an annealing temperature of 55°C and 30 cycles. The resultant 4-kb fragment was initially T/A cloned into the pGEM-T cloning vector (Promega, Madison, WI, USA) and sequenced. For heterologous expression in mammalian cells, SMDR2 was T/A cloned into the pcDNA3.1/V5-His-Topo vector (Invitrogen), placing it under control of the cytomegalovirus (CMV) promoter. The original SMDR2 construct in pGEM-T was used as a template, and the primers were SMDR2-3 and SMDR2-NS-R (TAGGCGGCCGCCTTTTGTATTATCTAATTTATATAA), a 3′ reverse primer that removes the stop codon at the end of the SMDR2 coding sequence, thereby allowing C-terminal tagging of the SMDR2 protein with the V5 epitope that is at the 3′ end of the inserts in the pcDNA3.1/V5-His-Topo vector. The high-fidelity Phusion enzyme (New England Biolabs, Ipswich, MA, USA) was used for 25 cycles of amplification according to the manufacturer’s instructions, with an annealing temperature of 68°C. Cloning and transformation were as recommended by the manufacturer, and several SMDR2 clones were sequenced.
Generation of a cell line stably transfected with SMDR2
Briefly, a day before the transfection, CHO-K1 cells were seeded in complete growth medium without antibiotics at 1 × 105 cells/cm2 in 6-well plates. The following day, cells were transfected with or without the SMDR2 (or empty vector) DNA construct by using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, the medium was replaced with selection medium containing G418 sulfate. After 2 wk, individual clones were selected by further dilution and amplification in 96-well plates.
Preparation of membrane vesicles
Cells were grown to 90% confluence, harvested using nonenzymatic cell dissociation solution (Sigma), and washed with Dulbecco’s PBS. The cell pellets were incubated in hypotonic buffer (10 mM Tris-HCl, 250 mM sucrose, 0.1 mM EDTA, and 1 mM DTT, pH 7.4) for 30 min at 4°C, and the cell lysate was homogenized with a polytron homogenizer (PT1200 E) on ice. The unlysed cells were removed by centrifuging at 1000 gmax for 10 min at 4°C, and the crude supernatant was layered slowly on top of a sucrose cushion (10 mM Tris-HCl and 35% sucrose, pH 7.4). After ultracentrifugation at 100,000 gmax for 1 h at 4°C, the interface layer containing the enriched membrane vesicles was collected and resuspended in suspension buffer (10 mM Tris-HCl and 250 mM sucrose, pH 7.4) by passing it through a 25-gauge needle repeatedly. The protein concentration was measured using a Bradford assay (Fermentas, Burlington, ON, Canada) with BSA (Sigma) as a standard, and samples were stored at −80°C until further use.
ATPase activity
The ATPase activity of SMDR2 membrane vesicles was determined using an ATPase assay kit (Innova Biosciences, Cambridge, UK) according to the manufacturer’s instructions, with minor modifications. Briefly, membrane vesicles from cells transfected with SMDR2 or empty vector or nontransfected parental cells were incubated 20–30 min at 37° in substrate buffer (0.5 M Tris-HCl, 0.1 M MgCl2, 10 mM purified ATP, 5 μM calcein-AM, 5 mM sodium azide, and 1 mM ouabain, pH 7.4) with or without Na-orthovanadate (2–20 μM). After incubation, the reaction was stopped by addition of 50 μl of Gold Mix (from the ATPase assay kit). Two minutes later, 20 μl of stabilizer (from the ATPase assay kit) was added and the solution was incubated for 20 min at 37°C in the dark. The enzyme activity was calculated by measuring the Pi-dye complex (yellow color) released from ATP hydrolysis using an ELISA plate reader at 620 nm (LabScan Multiscan Plus; LabScan, Dublin, Ireland).
Rhodamine uptake assay
To examine the transport properties of SMDR2, the rhodamine 123 (Invitrogen) uptake assay was performed as described previously (40), with some minor modifications. Membrane vesicles were attached to 96-well plates by incubation at 37°C for 30 min, and unbound vesicles were removed by rinsing with PBS. Vesicles were then pretreated with or without Pgp modulators (verapamil, nifedipine, and PZQ) for 30 min at 37°C and incubated in transport buffer (50 mM Tris-HCl, 0.25 M sucrose, 5 mM MgCl2, 2 mM ATP, 100 μg/ml creatine kinase, and 10 mM creatine phosphate) containing 20 μM rhodamine for 1 h at 37°C in the dark. After incubation, vesicles were washed twice with PBS and disrupted with the detergent 0.25% n-octyl-β-d-glucopyranoside (Sigma) for 10 min at 37°C to release the accumulated dye. Finally, rhodamine fluorescence was measured at room temperature using a fluorescence plate reader with an excitation of 485 nm and an emission of 535 nm.
Immunoblotting
Membrane vesicles (5 μg protein) were mixed with 1× NuPAGE LDS sample buffer (Invitrogen) and electrophoresed on a NuPAGE 4–12% Bis-Tris gel using 2-(N-morpholino)ethane sulfonic acid buffer. The proteins were transferred to nitrocellulose membrane using the Xcell II Blot Module (Invtirogen) and blocked with 5% nonfat dry milk powder in TBS-0.05% Tween 20 (TBST) for 3 h at room temperature. After being washed in TBST, the membrane was incubated overnight at 4°C with a mouse-monoclonal antibody against mammalian Pgp (C219; Abcam, Cambridge, MA, USA) in TBST plus 5% nonfat dry milk powder (1:500 dilution). This antibody targets two Pgp epitopes that are largely conserved in the SMDR2 sequence (see ref. 32 for details). After 3 washes in TBST, the blot was incubated with goat anti-mouse IgG antibody conjugated with horseradish peroxidase (Jackson Immunoresearch, West Grove, PA, USA) at a 1:10000 dilution for 1 h at room temperature. Blots were then washed thoroughly with TBST and exposed to X-ray film (Pierce, Rockford, IL, USA) after incubating in Super Signal West Pico Chemiluminescent substrate (Pierce). As a control for protein loading, the blot was reprobed with a mouse monoclonal antibody against a conserved N-terminal peptide of β-actin (clone AC-74; Sigma).
BODIPY-PZQ efflux assay
Fluorescent BODIPY-PZQ was synthesized and purified as described previously (12). Control parental CHO cells, or cells stably transfected with either SMDR2 or empty vector, were detached using 0.25% Trypsin-EDTA and washed 3 times with PBS. After being washed, the cells were preincubated with or without PZQ (15 μM) in balanced salt solution (140 mM NaCl, 2 mM CaCl2, 3 mM KCl, 30 mM glucose, and 25 mM HEPES/NaOH, pH 7.2) at 37°C for 30 min and loaded with fluorescent BODIPY-PZQ (1 μM). The cells were incubated for 30 min and washed twice in balanced salt solution with or without PZQ. Efflux of BODIPY-PZQ was measured in the FL-1 fluorescence channel on a FACSCalibur instrument (BD Biosciences, San Jose, CA, USA). As a control, efflux was measured in cells pretreated with a membrane-permeable form of BODIPY that is modified intracellularly to become membrane impermeable (CellTracker Green BODIPY, Invitrogen).
Fluorescence microscopy
Cells were loaded with fluorescent BODIPY-PZQ as described in the methods above for flow cytometry and observed under the compound microscope (Olympus BX61) equipped with Nomarski differential interference contrast optics and epifluorescence (Olympus America, Center Valley, PA, USA). Images were acquired with a Spot RT color digital camera and processed using Spot Advanced image analysis software package (Diagnostic Instruments, Sterling Heights, MI, USA) or Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA).
Statistics
Data were analyzed with GraphPad Prism (GraphPad, San Diego, CA, USA) or Excel (Microsoft, Redmond, WA, USA) and expressed as arithmetic means ± se. ANOVA or unpaired 2-tailed t tests, as appropriate, were used to assess significance. Values of P ≤ 0.05 were considered significant.
RESULTS
Stable transfection of SMDR2, a Pgp homologue from S. mansoni, into CHO cells
The sequence of the SMDR2 cDNA was originally reported by Bosch et al. (33). We used that sequence to design primers for amplification of the full-length SMDR2 coding region by RT-PCR from adult S. mansoni total RNA. The resultant ∼4-kb fragment was initially cloned into the pGem-T cloning vector and sequenced. All sequenced SMDR2 clones contained a disparity from the published sequence, consisting of a small frameshift resulting from insertion of an A after bp 2058 (published), a C at bp 2070, and a T at bp 2079. These changes result in an alteration of a 12-aa stretch in the protein sequence, plus an insertion of 1 aa, from LVELTILVFASL (published) to ISRAPNSLYLRLL. To confirm this difference, we repeatedly amplified this region of SMDR2, both by RT-PCR and by PCR of genomic DNA. In all cases, we obtained only the new sequence, including from amplified bands that were sequenced without cloning. In addition, an expressed sequence tag in the S. mansoni database (Sm05633) that matches the SMDR2 sequence also contains the sequence we found. Furthermore, the 13 residue stretch we found is more similar to MDR sequences from other species than is the published sequence (e.g., 5 conserved residues vs. 2 against human MDR, respectively). Based on this information, we believe that the SMDR2 sequence we obtained is in fact expressed in S. mansoni adults.
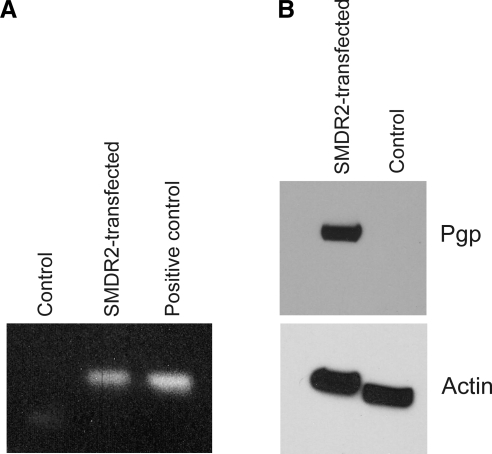
To explore the biochemical properties and substrate specificities of SMDR2 in a heterologous system, we generated CHO cells stably transfected with the SMDR2 coding region subcloned into the pcDNA3.1-V5-His-Topo vector, which places the sequence under the control of the CMV promoter. RT-PCR performed on RNA from these cells resulted in amplification of a single band of the expected size from the transfected cell line but not from control untransfected cells or cells transfected with empty vector (Fig. 1). Similarly, plasma membrane vesicles from the SMDR2-transfected CHO cell line, but not the G418-sensitive parental line, contain a 140- to 150-kDa protein recognized on Western blots by an anti-Pgp antibody (Fig. 1; in a single blot, we detected very faint, presumably endogenous, anti-Pgp immunoreactivity in the control cells). Taken together, these results clearly show that SMDR2 is expressed in the stably transfected cell line at both the mRNA and protein level. In addition, the parental, untransfected cell line expresses nearly undetectable levels of native Pgp.
Figure 1.
Expression of SMDR2 in stably transfected CHO cells. A) RT-PCR amplification of SMDR2 from CHO cells stably transfected with SMDR2 or the G418-sensitive parental control line. SMDR2 template was used as a positive control. B) Western blot analysis of Pgp or β-actin from membrane vesicles isolated from SMDR2-tranfected CHO cells or the parental control line. Anti-Pgp monoclonal antibody (C-219) recognizes a 140- to 150-kDa protein from the SMDR2-transfected CHO cell line but not from the parental control line.
Membrane vesicles from SMDR2-transfected CHO cells exhibit vanadate-sensitive ATPase activity
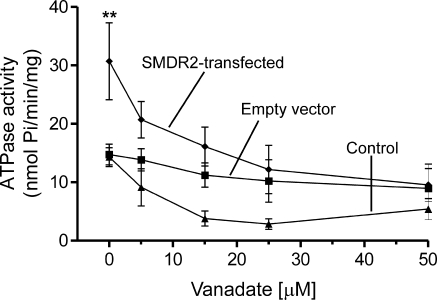
Mammalian Pgp exhibits vanadate-sensitive, substrate-stimulated ATPase activity (41). To characterize the biochemical properties of the SMDR2 transporter, we analyzed the substrate (calcein-AM)-stimulated ATPase activity of inside-out membrane vesicles by measuring the release of inorganic phosphate via ATP hydrolysis. Other ATPases, such as Na+-K+-ATPase and Ca2+-dependent ATPase, were blocked by including ouabain and EGTA in the assay buffer. As shown in Fig. 2, SMDR2-transfected vesicles display significantly higher ATPase activity than control untransfected vesicles or vesicles transfected with empty vector. SMDR2-transfected vesicles exhibit 30.68 ± 6.56 nmol Pi/min/mg protein of ATPase-specific activity, compared with 14.3 ± 1.58 and 14.75 ± 1.77 nmol/min/mg protein for control untransfected vesicles and empty vector-transfected vesicles, respectively. Vanadate, a potent inhibitor of ABC transporters (41), was tested on SMDR2 ATPase activity by incubating membrane vesicles with or without vanadate at different concentrations. As shown in Fig. 2, the inclusion of vanadate in the assay buffer significantly (P<0.01) blunts SMDR2 ATPase activity in a concentration-dependent manner. Taken together, these data show that SMDR2, like other Pgps, exhibits vanadate-sensitive ATPase activity.
Figure 2.
Vanadate-sensitive ATPase activity of SMDR2-transfected vesicles. Inside-out membrane vesicles were incubated in substrate buffer containing calcein-AM with or without Na-orthovanadate (0–20 μM) for 20–30 min at 37°C and assessed for ATPase activity. SMDR2-transfected vesicles (diamonds) show higher ATPase activity compared with vesicles transfected with empty vector (squares) or control untransfected cells (triangles). Inclusion of vanadate (2–50 μM) in substrate buffer significantly inhibited substrate-stimulated ATPase activity in a dose-dependent manner. **P ≤ 0.01.
Pgp modulators and PZQ inhibit rhodamine accumulation in SMDR2-transfected membrane vesicles
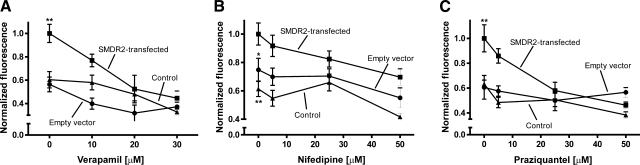
Rhodamine 123 is a fluorescent Pgp substrate, widely used as a reporter dye to study multidrug transporters (42). In whole-cell-based assays of Pgp activity, rhodamine is extruded across the cell membrane in an ATP-dependent manner. In contrast, in inside-out membrane vesicles, in which the substrate and ATP binding site are exposed to the bath solution, rhodamine transport by Pgp results in accumulation within the vesicles. Based on this principle, inside-out membrane vesicles were isolated from stable cell lines or control cells and incubated with rhodamine, and its accumulation was measured as described previously (40). As shown in Fig. 3, uptake of rhodamine is significantly higher in SMDR2-transfected vesicles than in vesicles from control cells (P<0.01) or those transfected with empty vector (P<0.01 or P<0.05). This observation correlates with the expression of the SMDR2 protein identified by immunoblot.
Figure 3.
Pgp modulators inhibit SMDR2-dependent rhodamine accumulation. Inside-out membrane vesicles were incubated in transport buffer containing rhodamine, a fluorescent Pgp substrate, in the presence or absence of verapamil (10–30 μM; A), nifedipine (5–50 μM; B), or PZQ (5–50 μM; C) for 30 min at 37°C. Influx of rhodamine is higher in membrane vesicles isolated from SMDR2-transfected CHO cells (squares) in comparison with empty vector (circles) or parental control cells (triangles), and that activity (normalized) is inhibited in the presence of the Pgp modulators verapamil (A) and nifedipine (B), as well as PZQ (C). *P ≤ 0.05, SMDR2-transfected vs. empty vector-transfected cells; **P ≤ 0.01, SMDR2-transfected vs. control, untransfected cells.
To define the pharmacological sensitivity of SMDR2, we performed additional rhodamine uptake experiments in the presence of verapamil (10–30 μM) and nifedipine (5–50 μM), L-type voltage-gated calcium (Cav) channel blockers that are also modulators of mammalian Pgp (42). Both compounds significantly inhibit rhodamine accumulation in SMDR2-transfected vesicles in a concentration-dependent manner (Fig. 3A, B; P<0.001, n=6, and P<0.05, n=5, respectively; comparison of maximal inhibition to no inhibitor added).
We have recently shown that exposure of adult schistosomes to sublethal concentrations of the antischistosomal drug PZQ modulates the expression of SMDR2 by transiently increasing the level of SMDR2 RNA and protein (32). In addition, PZQ has been shown by others to be an inhibitor of mammalian Pgp (38). To further explore the interplay between PZQ and SMDR2, we examined the effect of PZQ on SMDR2 activity using the membrane-based rhodamine accumulation assay. PZQ significantly (P<0.01) inhibited rhodamine accumulation in a dose-dependent manner (Fig. 3C) but had no effect in vesicles isolated from control cells or those transfected with empty vector. Thus, like its mammalian counterpart, SMDR2 is sensitive to modulation by PZQ.
There is a clearcut difference in the half-maximum inhibition of rhodamine transport by these 3 compounds, likely representative of differential levels of affinity for SMDR2. Based on the calculated IC50 values for verapamil (12.1±1.62 μM) and PZQ (17.4±1.16 μM) and the level of maximal inhibition, the relative inhibitory potency of these compounds on SMDR2 activity is verapamil > PZQ > nifedipine (we were not able to resolve an IC50 value for nifedipine from our data).
BODIPY-PZQ is a substrate of SMDR2
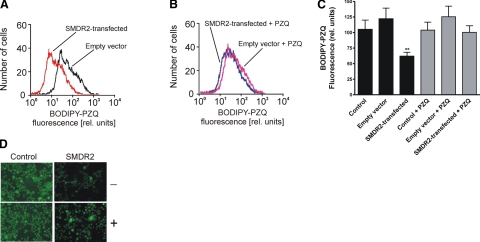
To test whether PZQ is a substrate for SMDR2, we loaded control and SMDR2-transfected cells with BODIPY-PZQ, a fluorescent derivative of PZQ (Fig. 4; ref. 12), and analyzed the efflux of the fluorophore using flow cytometry. As shown in Fig. 5, within 15 min, SMDR2-transfected cells, but neither untransfected cells nor cells transfected with empty vector, exhibited significant (P<0.01) efflux of the fluorescent PZQ derivative. The pretreatment of SMDR2-transfected cells with nonfluorescent PZQ (15 μM) significantly prevented efflux of the fluorophore (Fig. 5B). Efflux of a membrane-permeable form of BODIPY that is modified intracellularly to become membrane impermeable is not increased in SMDR2-transfected cells compared with control cells (data not shown). These results indicate that PZQ is a substrate for the membrane transporter SMDR2.
Figure 4.
Structure of PZQ and BODIPY-PZQ. Structure of PZQ and the fluorescent BODIPY-PZQ used in these experiments. Details on synthesis and properties of BODIPY-PZQ have been published previously (12).
Figure 5.
Efflux of fluorescent BODIPY-PZQ by SMDR2 in stably transfected cells. Cells expressing SMDR2 show higher efflux of BODIPY-PZQ that was significantly reduced in the presence of unlabeled PZQ. A) Flow cytometry histogram showing BODIPY-PZQ fluorescence in SMDR2-transfected cells (red) or cells tranfected with empty vector (black). B) FACS histogram showing effect of unlabeled PZQ on BODIPY-PZQ fluorescence in SMDR2-transfected cells (blue) or cells tranfected with empty vector (red). C) Pretreatment of cells with PZQ eliminates the difference in exclusion rate between SMDR2-expressing cells and parental control cells or cells transfected with empty vector. Mean BODIPY-PZQ fluorescence intensity of cells incubated for 30 min in balanced salt solution in the absence (black bars; n=10) or presence (gray bars) of 15 μM PZQ (n=10–11). Fluorescence intensity in SMDR2-expressing cells is significantly lower than in all other samples, including SMDR2-expressing cells with added 15 μM unlabeled PZQ. D) Micrographs of BODIPY-PZQ fluorescence in CHO cells in the absence (−) or presence (+) of 15 μM unlabeled PZQ. Control, untransfected cells; SMDR2, cells stably transfected with SMDR2. Concentration of BODIPY-PZQ in all cases was 1 μM. Note lower intracellular fluorescence in SMDR2-transfected cells compared with controls, indicating enhanced efflux of BODIPY-PZQ, and the increased fluorescence in those cells in the presence of unlabeled PZQ. **P ≤ 0.01; unpaired t test.
DISCUSSION
ABC transporters such as Pgp play crucial roles in the efflux of toxic and xenobiotic compounds, including chemotherapeutics, from cells (18, 19). These proteins mediate MDR in mammalian cells, are implicated in drug resistance in nematodes (29, 30), and have been proposed as candidate targets for potentiation of anthelmintic activity of other drugs (25). In this study, we show that SMDR2, a Pgp-like protein from S. mansoni, encodes a functional efflux transporter and is inhibited by Pgp modulators. Furthermore, we demonstrate that the antischistosomal drug PZQ acts as both a substrate and an inhibitor of SMDR2.
The interaction of PZQ with SMDR2 is particularly intriguing in light of recent findings (32) showing that exposure of schistosomes to sublethal PZQ concentrations in vitro results in a transient increase in expression of SMDR2 RNA and protein in adult male parasites and, furthermore, that a schistosome isolate with reduced PZQ susceptibility expresses increased levels of SMDR2. PZQ also alters worm excretion of resorufin, a likely Pgp substrate (36). These interactions of PZQ with the schistosome efflux transport system could provide strategies for potentiating the effects of PZQ and may offer clues to the development of drug resistance in schistosomes.
Interestingly, a relatively high concentration (∼100 μM) of verapamil has been reported to suppress egg production in S. mansoni, while a lower concentration (∼5 μM) appears to delay the initiation of egg production in vitro (39, 43). Similarly, both verapamil (∼100 μM) and nifedipine (∼70 μM) suppress egg production in another trematode, Echinostoma caproni. These results have been interpreted to result from inhibition of flatworm Cav channels by these two Cav channel antagonists. However, invertebrate Cav channels typically show relatively low sensitivity to these agents (44). An alternative hypothesis, based on the results presented here, is that these drugs are instead (or in addition) interacting with SMDR2 or some other platyhelminth Pgp-like transporter.
Despite our findings that PZQ is a modulator of SMDR2, it is unlikely that SMDR2 is the primary molecular target of PZQ. The effects on SMDR2 expression (32) required hours of incubation in sublethal concentrations of the drug in vitro, in contrast to the action of PZQ on worms, which is very rapid. More likely, SMDR2 is involved in the removal of PZQ from cells within the worm. In vivo, PZQ may either be inhibiting or, as a substrate, overwhelming the schistosome SMDR2 efflux transporter, either of which might serve as a signal for cells to increase SMDR2 expression. PZQ has been shown by others (36, 37) to alter excretion of a Pgp substrate in schistosomes, which may be a functional representation in vivo of the effects we see on SMDR2 activity in the heterologous system. On the other hand, recent evidence (12) suggests that PZQ may be interfering with energy production in the cell, thereby reducing the ATP levels required for schistosome ABC transporters such as SMDR2 to function. Testing of PZQ on SMDR2 function within schistosome (or other platyhelminth) cells may help further clarify this question. It will also be interesting to determine whether PZQ interacts with other schistosome drug and xenobiotic transporters and additionally whether similar effects on SMDR2 modulation and expression can be found in worms residing within hosts treated with the drug. Further experiments to test whether SMDR2 modulators such as verapamil potentiate the action of PZQ on schistosomes could provide clues to practical ways to enhance the effectiveness of the drug or perhaps overcome drug resistance. The fact that PZQ is able to modulate SMDR2, as shown here, and that SMDR2 is expressed at higher levels in at least one schistosome isolate with reduced PZQ susceptibility (32), argues for the possibility that SMDR2 or other schistosome drug transporters might play a role in the development of resistance to PZQ. Pharmacological and genetic disruption of SMDR2 and other drug transporter function should provide further insights into the role of xenobiotic efflux transporters in the physiological responses of schistosomes to antischistosomal drugs.
Acknowledgments
We thank William Morgan, Christina Evola, and Jessica Roberts-Misterly for excellent technical assistance. We also thank Fred Lewis and the National Institute of Allergy and Infectious Diseases Schistosome Resource Center for supplying the schistosome life cycle. Flow cytometry experiments were performed at the Flow Cytometry Core Facility at the University of Pennsylvania School of Veterinary Medicine. This work was supported by National Institutres of Health (NIH) grants R01 AI-40522 and R01 AI-73660 (to R.M.G.). R.M.G. and S.M.M. were also supported in part by NIH/National Science Foundation Woods Hole Center for Oceans and Human Health grant WHOI-A100354/A100360. Support was also received from the NIH Biocurrents Research Center at the Marine Biological Laboratory (P41 RR-001395). This work was also supported in part by the American Lebanese Syrian Associated Charities, St. Jude Children’s Research Hospital.
References
- Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Tropica. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2:12–13. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Kusel J R, McVeigh P, Thornhill J A. The schistosome excretory system: a key to regulation of metabolism, drug excretion and host interaction. Trends Parasitol. 2009;25:353–358. doi: 10.1016/j.pt.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Andrews P. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol Ther. 1985;29:129–156. doi: 10.1016/0163-7258(85)90020-8. [DOI] [PubMed] [Google Scholar]
- Greenberg R M. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Greenberg R M. Ca2+ signalling, voltage-gated Ca2+ channels and praziquantel in flatworm neuromusculature. Parasitology. 2005;131:S97–S108. doi: 10.1017/S0031182005008346. [DOI] [PubMed] [Google Scholar]
- Doenhoff M J, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- Day T A, Bennett J L, Pax R A. Praziquantel: the enigmatic antiparasitic. Parasitol Today. 1992;8:342–344. doi: 10.1016/0169-4758(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Redman C A, Robertson A, Fallon P G, Modha J, Kusel J R, Doenhoff M J, Praziquantel Martin R J. An urgent and exciting challenge. Parasitol Today. 1996;12:14–20. doi: 10.1016/0169-4758(96)80640-5. [DOI] [PubMed] [Google Scholar]
- Jeziorski M C, Greenberg R M. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int J Parasitol. 2006;36:625–632. doi: 10.1016/j.ijpara.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon A D, Imani R A, Blackburn V R, Cupit P M, Melman S D, Goronga T, Webb T, Loker E S, Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol Biochem Parasitol. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F, Basso A, Bellelli A, Brunori M, Pica Mattoccia L, Valle C. The anti-schistosomal drug praziquantel is an adenosine antagonist. Parasitology. 2007;134:1215–1221. doi: 10.1017/S0031182007002600. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Salunkhe A M, Mallia A K, He Y X, Kalyanasundaram R. Praziquantel affects the regulatory myosin light chain of Schistosoma mansoni. Antimicrob Agents Chemother. 2009;53:1054–1060. doi: 10.1128/AAC.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallima H, El Ridi R. Praziquantel binds Schistosoma mansoni adult worm actin. Int J Antimicrob Agents. 2007;29:570–575. doi: 10.1016/j.ijantimicag.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L, Orsini T, Basso A, Festucci A, Liberti P, Guidi A, Marcatto-Maggi A L, Nobre-Santana S, Troiani A R, Cioli D, Valle C. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp Parasitol. 2008;119:332–335. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Day T A, Botros S. Drug resistance in schistosomes. Maule A, Marks N J, editors. Wallington, Oxfordshire, UK: CAB International; Parasitic FlatwormsMolecular Biology, Biochemistry, Immunology and Physiology. 2006:256–268. [Google Scholar]
- Leonard G D, Fojo T, Bates S E. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- Ambudkar S V, Kimchi-Sarfaty C, Sauna Z E, Gottesman M M. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- Seeger M A, van Veen H W. Molecular basis of multidrug transport by ABC transporters. Biochim Biophys Acta. 2009;1794:725–737. doi: 10.1016/j.bbapap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Aller S G, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell P M, Trinh Y T, Zhang Q, Urbatsch I L, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen I T. Multidrug efflux pumps and resistance: regulation and evolution. Curr Opin Microbiol. 2003;6:446–451. doi: 10.1016/j.mib.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Zhou S F, Wang L L, Di Y M, Xue C C, Duan W, Li C G, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- Valderramos S G, Fidock D A. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol Sci. 2006;27:594–601. doi: 10.1016/j.tips.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespine A, Alvinerie M, Vercruysse J, Prichard R K, Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Xu M, Molento M, Blackhall W, Ribeiro P, Beech R, Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol Biochem Parasitol. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- Sangster N C, Bannan S C, Weiss A S, Nulf S C, Klein R D, Geary T G. Haemonchus contortus: sequence heterogeneity of internucleotide binding domains from P-glycoproteins. Exp Parasitol. 1999;91:250–257. doi: 10.1006/expr.1998.4373. [DOI] [PubMed] [Google Scholar]
- Blackhall W J, Prichard R K, Beech R N. P-glycoprotein selection in strains of Haemonchus contortus resistant to benzimidazoles. Vet Parasitol. 2008;152:101–107. doi: 10.1016/j.vetpar.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Bourguinat C, Ardelli B F, Pion S D, Kamgno J, Gardon J, Duke B O, Boussinesq M, Prichard R K. P-glycoprotein-like protein, a possible genetic marker for ivermectin resistance selection in Onchocerca volvulus. Mol Biochem Parasitol. 2008;158:101–111. doi: 10.1016/j.molbiopara.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Kerboeuf D, Blackhall W, Kaminsky R, von Samson-Himmelstjerna G. P-glycoprotein in helminths: function and perspectives for anthelmintic treatment and reversal of resistance. Int J Antimicrob Agents. 2003;22:332–346. doi: 10.1016/s0924-8579(03)00221-8. [DOI] [PubMed] [Google Scholar]
- Jones P M, George A M. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. Int J Parasitol. 2005;35:555–566. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Messerli S M, Kasinathan R S, Morgan W, Spranger S, Greenberg R M. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol Biochem Parasitol. 2009;167:54–59. doi: 10.1016/j.molbiopara.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch I B, Wang Z X, Tao L F, Shoemaker C B. Two Schistosoma mansoni cDNAs encoding ATP-binding cassette (ABC) family proteins. Mol Biochem Parasitol. 1994;65:351–356. doi: 10.1016/0166-6851(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Sato H, Kusel J R, Thornhill J. Functional visualization of the excretory system of adult Schistosoma mansoni by the fluorescent marker resorufin. Parasitology. 2002;125:527–535. doi: 10.1017/s0031182002002536. [DOI] [PubMed] [Google Scholar]
- Sato H, Kusel J R, Thornhill J. Excretion of fluorescent substrates of mammalian multidrug resistance-associated protein (MRP) in the Schistosoma mansoni excretory system. Parasitology. 2004;128:43–52. doi: 10.1017/s0031182003004177. [DOI] [PubMed] [Google Scholar]
- Oliveira F A, Kusel J R, Ribeiro F, Coelho P M. Responses of the surface membrane and excretory system of Schistosoma mansoni to damage and to treatment with praziquantel and other biomolecules. Parasitology. 2006;132:321–330. doi: 10.1017/S0031182005009169. [DOI] [PubMed] [Google Scholar]
- Kusel J R, Oliveira F A, Todd M, Ronketti F, Lima S F, Mattos A C, Reis K T, Coelho P M, Thornhill J A, Ribeiro F. The effects of drugs, ions, and poly-l-lysine on the excretory system of Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2006;101:293–298. doi: 10.1590/s0074-02762006000900046. [DOI] [PubMed] [Google Scholar]
- Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell A L. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Walter M, Kuris A. Methods for the inhibition of egg production in trematodes. 2003 U.S. Patent 6,514,963 B2. [Google Scholar]
- Kohler S, Stein W D. Optimizing chemotherapy by measuring reversal of P-glycoprotein activity in plasma membrane vesicles. Biotechnol Bioeng. 2003;81:507–517. doi: 10.1002/bit.10488. [DOI] [PubMed] [Google Scholar]
- Senior A E, al-Shawi M K, Urbatsch I L. The catalytic cycle of P-glycoprotein. FEBS Lett. 1995;377:285–289. doi: 10.1016/0014-5793(95)01345-8. [DOI] [PubMed] [Google Scholar]
- Shapiro A B, Ling V. The mechanism of ATP-dependent multidrug transport by P-glycoprotein. Acta Physiol Scand Suppl. 1998;643:227–234. [PubMed] [Google Scholar]
- Bonn D. Schistosomiasis: a new target for calcium channel blockers. Lancet Infect Dis. 2004;4:190. doi: 10.1016/s1473-3099(04)00986-7. [DOI] [PubMed] [Google Scholar]
- Jeziorski M C, Greenberg R M, Anderson P A. The molecular biology of invertebrate voltage-gated Ca2+ channels. J Exp Biol. 2000;203:841–856. doi: 10.1242/jeb.203.5.841. [DOI] [PubMed] [Google Scholar]