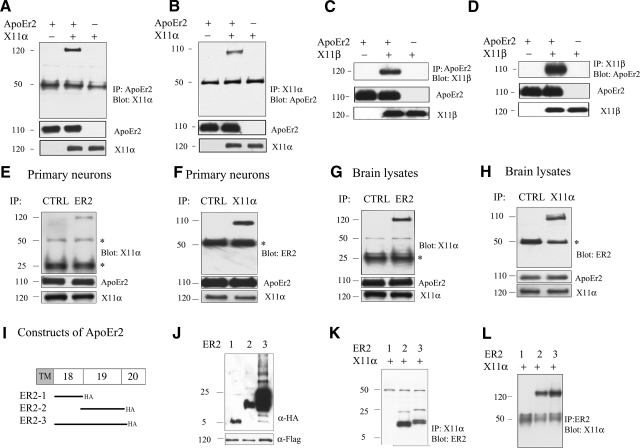
Figure 1.
X11α and X11β interact with ApoEr2 in COS7 cells, in primary neurons, and brain, via exon 19 of ApoEr2. A, B) COS7 cells were transiently transfected with ApoEr2-HA, ApoEr2-HA and X11α-Flag, or X11α-Flag. Cell lysates were immunoprecipitated with HA and probed with anti-Flag (n=3) (A) or immunoprecipitated with anti-Flag and probed with anti-HA (n=3) (B). X11α and ApoEr2 coprecipitated. C, D) COS7 cells were transfected with ApoEr2, ApoEr2 and X11β, or X11β. Cell lysates were immunoprecipitated with anti-HA and probed with anti-Flag (n=3) (C), or immunoprecipitated with anti-Flag and probed with anti-HA (n=3) (D). ApoEr2 and X11β coprecipitated. Immunoblot of cell lysates showed similar levels of ApoEr2 and X11α or ApoEr2 and X11β (middle and bottom panels). E, F) Primary cortical neurons were immunoprecipitated with anti-ApoEr2 and probed with anti-X11α (E), or with anti-X11α and probed with anti-ApoEr2 (n=3) (F). As a negative control, the experiment was conducted with an irrelevant antibody (CTRL). G, H) Mouse brain lysates were immunoprecipitated with ApoEr2 antibody and probed with anti-X11α (G), or with X11α antibody and probed with anti-ApoEr2 (n=3) (H). An irrelevant antibody was used as negative control (CTRL). Bottom panels show total levels of proteins in lysates. Asterisks denote IgG heavy/light chains. I) Constructs of ApoEr2 with C-terminal HA tags containing exon 18 (ER2-1), exon 19 (ER2-2), and exon 18 and 19 (ER2-3). J) Cell lysates from COS7 cells transfected with indicated constructs were probed with anti-HA to demonstrate protein expression. K, L) COS7 cells were transfected with full-length X11α and ApoEr2 constructs as indicated. Cell lysates were immunoprecipitated with anti-Flag antibody and probed with anti-HA antibody (n=2) (K) or immunoprecipitated with anti-HA antibody and probed with anti-Flag antibody (n=2) (L).