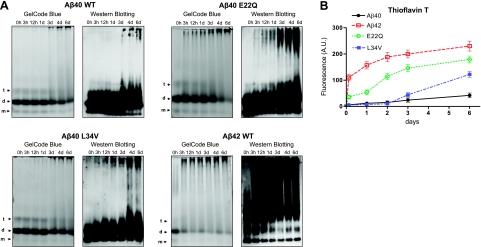
Figure 1.
Comparative study of structural properties of Aβ genetic variants. HFIP-treated peptides at 1 mg/ml in PBS, prepared as described in Materials and Methods, were incubated at 37°C for up to 6 d, and their aggregation/fibrillization propensity was analyzed by native gel electrophoresis (A) and Thioflavin T binding (B). A) Left panels: GelCode Blue protein staining after separation on nondenaturing 10–30% gradient polyacrylamide gels. Arrows indicate the positions of monomers (m), dimers (d), and tetramers (t). Right panels: Western blot analysis subsequent to nondenaturing electrophoresis probed with a mixture of 4G8 and 6E10 antibodies. B) Thioflavin T binding assay. Fluorescence evaluation (excitation and emission wavelengths 435 and 490 nm, respectively) of Thioflavin T binding assay of the samples collected at the different time points during the 6-d duration of the experiments was performed as described in Materials and Methods. Data are means ± sd of duplicate independent experiments. AU, arbitrary units.