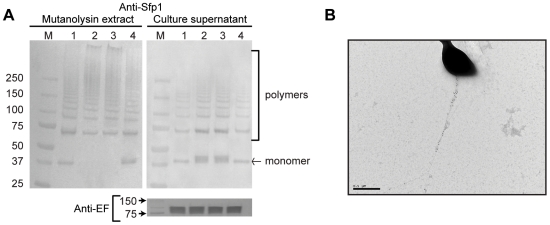
Figure 5. Role of srtA in formation of the pilus encoded by the srtF cluster.
A) Western-blotting analysis using anti-Sfp1 antisera of cell wall-anchored proteins (left) and concentrated culture supernatants (right) of S. suis strain P1/7 (lane 1), its derived ΔsrtA mutant (lane 2), a mock-complemented ΔsrtA mutant (lane 3) and the ΔsrtA mutant complemented in trans with srtA (lane 4) (upper panel). SDS-PAGE and Western-blotting were performed as described in Figure 2 legend. While Sfp1 polymers were detected in the cell wall fraction of all four tested strains, Sfp1 monomers were absent from the ΔsrtA mutant and the mock-complemented ΔsrtA. Reintroduction of the srtA gene into the ΔsrtA mutant complemented the defect (left upper panel). More Sfp1 monomers and slightly more Sfp1 polymers were detected in the culture supernatant of the ΔsrtA mutant, while the WT phenotype was restored by complementation with the srtA gene (right upper panel). To ensure that equal amounts of protein were loaded per well in the supernatant fraction, detection of the previously reported [68] secreted protein extracellular factor (EF) was carried out using the same samples and a previously described monoclonal antibody [55] (lower panel). M: Molecular weight markers. B) Immunogold labeling and transmission electron microscopy (magnification: 20,000×) showing scarce but very long pilus-like structures on the cell surface of the ΔsrtA mutant. Note the differences in magnification with the microphotographs shown in Figure 4.