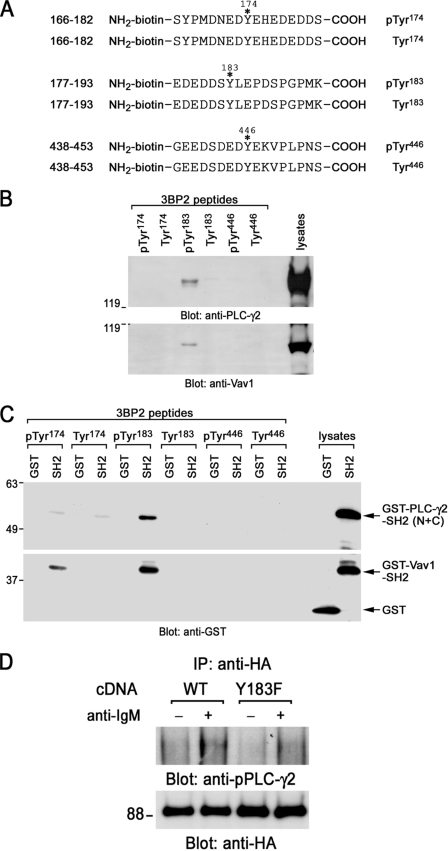
FIGURE 5.
Interaction of 3BP2 with PLC-γ2 and Vav1. A, shown is a schematic diagram of biotinylated synthesized 3BP2 peptides used in this experiment. Phosphorylated or non-phosphorylated 3BP2 peptides Ser166–Ser182, Glu177–Lys193, and Gly438–Ser453 are shown. Positions of phosphorylation sites are indicated with asterisks. Tyr174, Tyr183, or Tyr446 was phosphorylated in each phosphorylated 3BP2 peptide, respectively. These tyrosine residues were not phosphorylated in non-phosphorylated 3BP2 peptides. B, affinity purification by peptide binding experiments is shown. Daudi cell lysates were reacted with either phosphorylated or non-phosphorylated biotinylated synthesized 3BP2 peptides prebound to streptavidin beads. Interactions of these peptides with PLC-γ2 and Vav1 were analyzed by SDS-PAGE and immunoblotting with anti-PLC-γ2 and anti-Vav1 antibodies. C, interaction of 3BP2 peptides with GST-SH2 domain fusion proteins is shown. Bacterial GST, GST fusion proteins of PLC-γ2-SH2 (N+C), or Vav1-SH2 was reacted with each 3BP2 peptide prebound to streptavidin beads. Interactions of these peptides with SH2 domains were analyzed by SDS-PAGE and immunoblotting with anti-GST mAb. The last two lanes show the positive controls of GST and GST-SH2 fusion proteins of PLC-γ2 (N+C) and Vav1. D, shown is a co-immunoprecipitation experiment. Ramos-T cells were transiently transfected with HA-Myc-3BP2 wild type (WT) and HA-myc-3BP2-Y183F (Y183F). After 24 h cells were unstimulated (−) or stimulated with anti-IgM mAb for 3 min (+). Anti-HA immunoprecipitates (IP) were separated by SDS-PAGE and analyzed by immunoblotting with anti-phospho-PLC -γ2 (upper panel) and anti-HA antibodies (bottom panel). Molecular size markers are indicated at the left in kilodaltons. The results are representative of three independent experiments.