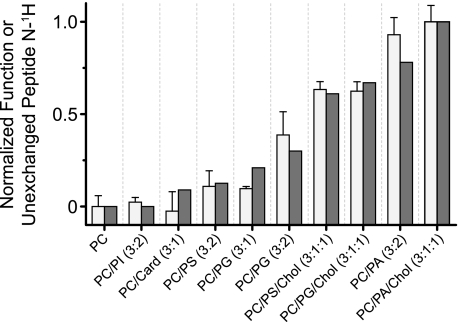
FIGURE 2.
Ability of the nAChR to undergo agonist-induced allosteric transitions is correlated with reduced nAChR solvent accessibility. Solvent accessibility was assessed from the extent of nAChR peptide hydrogen/deuterium exchange in each membrane environment (white bars), as measured from the residual amide II band in spectra recorded after 72 h in 2H2O at 4 °C (supplemental Figs. S1 and S2). The relative abilities of the nAChR to undergo agonist-induced resting-to-desensitized transitions was determined from the relative intensities of the conformationally sensitive difference band centered at 1655 cm−1 (gray bars) observed in Carb difference spectra recorded from each reconstituted membrane (Fig. 3). Both nAChR activity/function and the extent of unexchanged hydrogens were normalized to the values obtained with PC-nAChR (0) and PC/PA/Chol-nAChR (1.0). For PC-nAChR, ∼20% of the peptide hydrogens are resistant to exchange after 72 h in 2H2O at 4 °C. For the PC/PA/Chol-nAChR, ∼40% of the peptide hydrogens are resistant to peptide hydrogen/deuterium exchange. The error bars for the extent of hydrogen/deuterium exchange are the mean ± S.E. for n = 2–5 measurements.