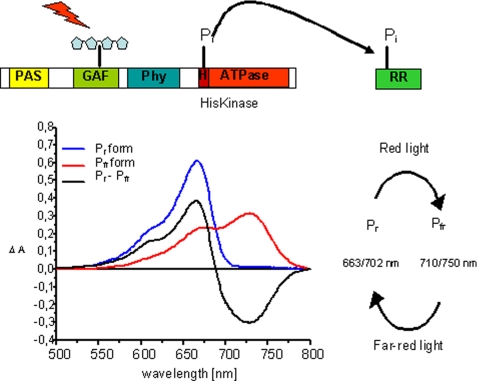
SCHEME 1.
Schematic presentation of the prokaryotic phytochrome domain structure and model for a HK activity. Phytochromes bind their chromophore in the N-terminal domain; shown here is the canonical situation for phytochromes where the chromophore is bound to a cysteine in the GAF domain (realized in this study with CphA). Some bacterial phytochromes (realized here with CphB) bind biliverdin as chromophore at a cysteine in the PAS domain. The histidine kinase activity is located in both types of phytochromes in the C-terminal domain. Upon excitation, HK utilizes a bound ATP molecule and phosphorylates a conserved histidine residue. Upon protein-protein contact between the HK domain and its response regulator, the phosphate group is transferred to a conserved aspartate residue in the RR protein. Also shown (bottom left) are the absorption spectra of the two forms of phytochrome, Pr and Pfr, the difference spectrum Pr − Pfr, and (bottom right) a scheme indicating the red and far-red light-induced Pr and Pfr formation, together with the λmax values of the respective states (Pr/Pfr). Wavelengths for PCB-assembled phytochromes are given first, followed by those for BV-assembled phytochromes.