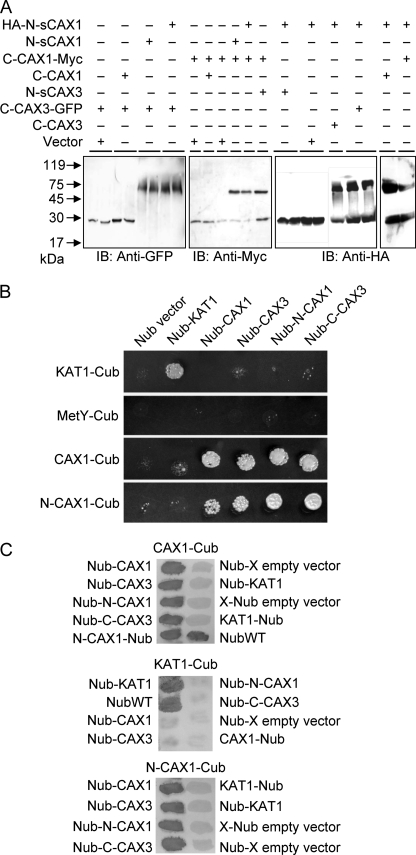
FIGURE 4.
Yeast protein interaction assays. A, physical interaction in yeast of N- and C-terminal halves of CAX1 and CAX3 by gel mobility shift assay. The proteins were isolated and solubilized from yeast cells co-expressing HA-tagged sCAX1 N-terminal half (HA-N-sCAX1), sCAX1 C-terminal half (C-CAX1-Myc), or CAX3 C-terminal-GFP fusion (C-CAX3-GFP) with tagged or nontagged sCAX1 N-terminal half (HA-N-sCAX1 or N-sCAX1), CAX1 C-terminal half (C-CAX1-Myc, C-CAX1), CAX3 C-terminal half (C-CAX3 or C-CAX3-GFP), sCAX3 N-terminal half (N-sCAX3), or empty vectors. Following 12% SDS-PAGE and transfer to polyvinylidene difluoride membranes, single tagged split half proteins or protein complexes were detected with immunoblotting (IB) using c-Myc, HA, and GFP antibodies. B, split ubiquitin interaction growth assay. Interaction between CAX1 and CAX3 was determined by growth assay on synthetic minimal medium lacking all six amino acids but supplemented with 150 μm Met for 3 days. The empty Cub and Nub vectors were used as a negative control, and the K+ channel KAT1 was used as a positive control. CAX1-Cub, N-CAX1-Cub, KAT1-Cub, or empty Cub vector (MetY-Cub) were bait for interaction in combination with empty Nub vector, Nub-KAT1, Nub-CAX1, Nub-CAX3, Nub-N-CAX1, and Nub-C-CAX3, respectively. All of the experiments were repeated at least three times with similar results obtained in each replicate. C, X-gal filter assay. Yeast filters set on synthetic minimal medium lacking Ade and His but supplemented with 150 μm Met were grown for 3 days then incubated for 30 min in X-gal solution. All of the experiments were repeated at least three times with similar results obtained in each replicate. The interaction assays are shown with CAX1-Cub (top panel), KAT1-Cub (middle panel), or N-CAX1-Cub (bottom panel) as bait construct and tested in combination with the various Nub-containing prey constructs.