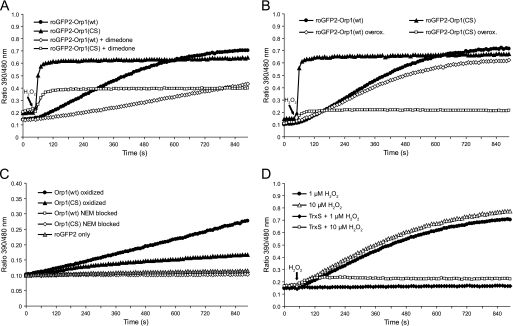
FIGURE 3.
Mechanism of Orp1-mediated roGFP2 oxidation. A, dimedone attenuates the response of both roGFP2-Orp1(wt) and roGFP2-Orp1(CS). Reduced fusion proteins were treated with 1 μm H2O2 in the presence or absence of 50 mm dimedone. B, roGFP2-Orp1(CS), but not roGFP2-Orp1(wt), is susceptible to overoxidation (overox.). Reduced fusion proteins were exposed to 5 mm H2O2 for 30 min, desalted, exposed to DTT (10 mm, 20 min), and desalted again. The fusion proteins (1 μm) were then treated with 10 μm H2O2. Untreated fusion proteins served as controls. C, the disulfide form of Orp1 is capable of mediating roGFP2 oxidation. Wild type and mutant Orp1 proteins were oxidized with a 5-fold molar excess of H2O2 or blocked with N-ethyl maleimide (NEM) followed by desalting. 1 μm reduced roGFP2 was incubated with 50 μm pretreated Orp1(wt) or Orp1(CS). D, thioredoxin competes thiol-disulfide exchange between roGFP2 and wild type Orp1. roGFP2-Orp1(wt) was exposed to 1 or 10 μm H2O2 in the presence or absence of a functional TrxS consisting of Trx1, Trx reductase, and NADPH. In A, B, and D, H2O2 was injected after 50 s.