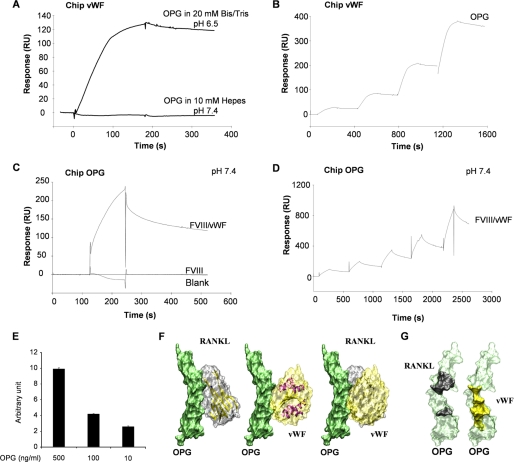
FIGURE 2.
The interaction between OPG and the FVIII·vWF complex occurred through the vWF. A, OPG binds to immobilized-vWF chip in specific biochemical conditions. Binding assays were performed using 2 different buffers (pH 7.4 or 6.5) as described under “Experimental Procedures.” B, determination of the Kd of OPG for vWF using a single cycle kinetic assay is shown. OPG was injected over immobilized vWF in pH 6.5 buffer at 25 nm and then ½ dilutions. C, in the pH 7.4 buffer, FVIII·vWF complex, but not recombinant Factor VIII, binds to OPG. FVIII·vWF complex (50 units/ml) or recombinant Factor VIII (50 units/ml) was injected at a flow rate of 30 μl/min over the immobilized-OPG sensor chip for 5 min, and the dissociation was monitored for 10 min. D, determination of the Kd of OPG for FVIII·vWF complex in pH 7.4 buffer using a single cycle kinetic assay is shown. FVIII·vWF complex was injected over immobilized-OPG starting at 300 nm and then ½ dilutions. E, OPG (500, 100, and 10 ng/ml) bound to the coated FVIII·vWF complex (1 unit/ml) using an ELISA assay is shown. Results are expressed using arbitrary units. F, modeling of the interactions between OPG (green), RANKL (gray), and the A1 domain of vWF (yellow) is shown. OPG has the same orientation in the three illustrations. The right illustration is an overlay of left and middle illustrations. G, shown is a representation of the binding domains involved in the interaction OPG-RANKL and OPG-vWF. OPG amino acids involved for the binding with RANKL are schematized in black, and those involved for the binding with vWF are schematized in yellow.