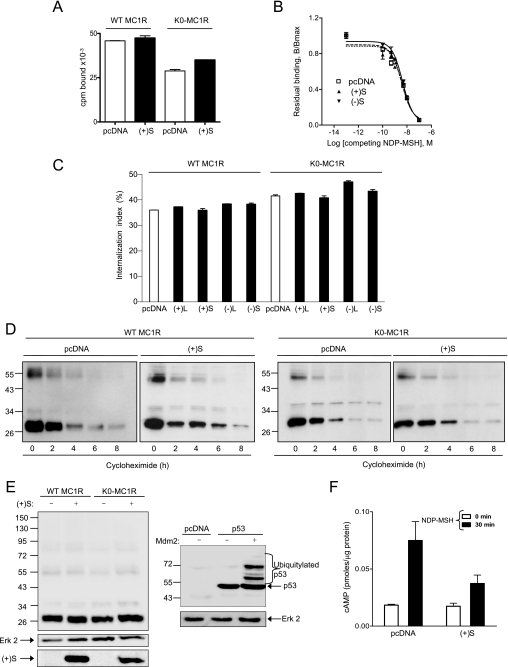
FIGURE 3.
Effects of MGRN1 on plasma membrane availability and intracellular stability of MC1R and inhibition of functional coupling of the K0-MC1R ubiquitylation null MC1R mutant. A, lack of down-regulation of NDP-MSH binding sites by MGRN1. HEK cells were transfected with WT or K0-MC1R and with (+)S MGRN1 (closed bars) or empty pcDNA3 (open bars). Cells were incubated with 10−10 m [125I]NDP-MSH for 1 h, washed, and bound radioactivity was counted. B, competition binding analysis for MC1R in the presence of two MGRN1 variants. Cells expressing MC1R alone or with (+)S or (−)S were incubated with 10−10 m [125I]NDP-MSH and increasing concentrations of unlabeled ligand, as indicated. The specifically bound radioactivity was measured. Results are given as % maximal binding in the absence of competitor. C, effect of MGRN1 isoforms on MC1R internalization. HEK293T cells were transfected with WT or K0-MC1R and the indicated MGRN1 isoforms (closed bars) or empty pcDNA as control (open bars). Transfected cells were incubated with [125I]NDP-MSH for 1.5 h. The amount of internalized radioligand was determined by an acid-wash procedure and an internalization index (percentage of agonist internalized relative to total binding) was calculated. D, effect of (+)S on the intracellular stability of MC1R. HEK293T cells expressing FLAG-tagged WT or K0-MC1R with or without (+)S MGRN1 were treated with 10−4 m cycloheximide and harvested at the times shown. Solubilized cell extracts were electrophoresed and immunoblotted with αFLAG. A representative blot is shown out of three experiments with similar results. E, comparable electrophoretic pattern of WT and K0-MC1R. HEK293T cells transfected to express WT or mutant MC1R with or without Myc epitope-labeled (+)S MGRN1 were analyzed for MC1R by Western blot (left blot, upper). Blots were stripped and probed for ERK2 as a loading control (left blot, middle), and for MGRN1 as a control for comparable expression of (+)S (left blot, lower). As a positive control for recovery and detection of the ubiquitylated species, HEK cells were transfected with empty vector or FLAG-labeled p53 alone or with its E3 Ub ligase Mdm2. Cell extracts were analyzed for p53 to detect ubiquitylated forms (right blot, upper) and for ERK2 (right gel, lower) as a loading control. F, functional coupling of K0-MC1R in the presence or absence of (+)S MGRN1. HEK293T transfected with K0-MC1R and empty vector (pcDNA) or (+)S were stimulated (10−7 m NDP-MSH, 30 min), and cAMP was measured. Empty bars correspond to vehicle-treated controls and filled bars to agonist-stimulated cells. Error bars represent S.D., n > 5.