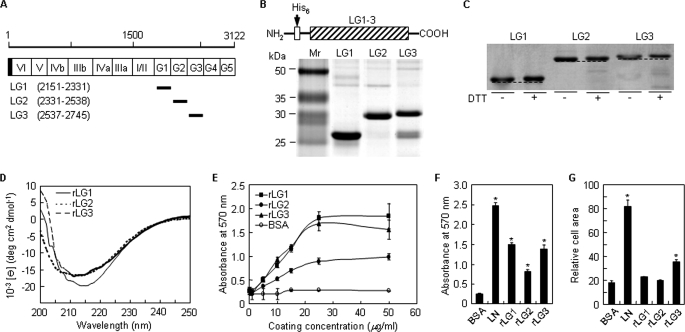
FIGURE 1.
Analyses of purified rLG proteins of human laminin α2 chain by SDS-PAGE and circular dichroism and their cell adhesion activities. A, schematic diagram of the human laminin α2 LG domains and its recombinant proteins. The amino acid scale is shown on the top, and the domain structures of the laminin α2 chain are indicated with open column. The shaded portion and the closed bars represent the signal peptide and the positions of the recombinant proteins, respectively. Numbers in parentheses correspond to the amino acid positions of the recombinant proteins in the entire laminin α2 chain. B, schematic diagram and SDS-PAGE analysis of the rLG proteins in the human laminin α2 chain. The three rLG proteins were expressed as His6-tagged fusion proteins. The rLG proteins were subjected to SDS-PAGE analysis (13% polyacrylamide gels, reducing) and visualized by Coomassie staining. C, gel mobilities of purified rLG proteins treated with dithiothreitol (DTT) were compared with nontreated rLG proteins under 10% SDS-PAGE conditions. Reduction of rLG proteins prior to electrophoresis resulted in reproducible decrease in gel mobility. D, CD analyses of rLG proteins in PBS, pH 3.0, at 23 °C. The CD spectra of all three rLG proteins showed ellipticity minima at around 210–220 nm, indicating that the purified rLG proteins consist predominantly of β-structure. E, rLG proteins support adhesion of PC12 cells seeded on rLG protein-coated plates for 1 h in serum-free medium in a dose-dependent manner. F, adhesion of PC12 cells seeded on plates coated with laminin (LN; 5 μg/ml) and rLG proteins (25 μg/ml) for 1 h in serum-free medium. G, cell spreading to laminin and rLG proteins. PC12 cells were seeded on laminin (LN; 5 μg/ml)- or rLG protein (25 μg/ml)-coated plates for 3 h in serum-free medium. Cell area was measured using Image-Pro Plus software. Values are expressed as the mean ± S.D. (n = 4). *, p < 0.01.