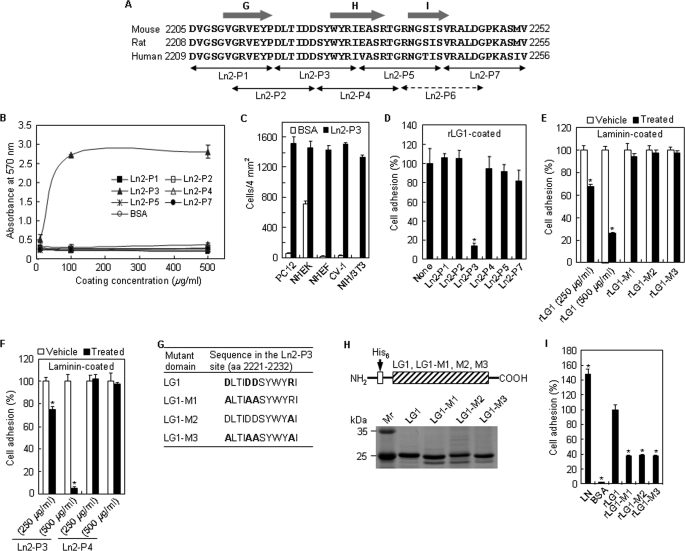
FIGURE 2.
Novel motif DLTIDDSYWYRI (Ln2-P3) within the LG1 domain promotes cell adhesion. A, amino acid sequence alignment of part of the laminin α2 LG1 domain from humans, rats, and mice. The arrows indicate the locations of the synthetic peptides. The predicted β-strand structures are represented by the gray arrows. B, dose-dependent cell adhesion to immobilized synthetic peptides. The synthetic peptides were coated onto plates by drying them for 12 h at room temperature, and PC12 cells were allowed to adhere for 1 h in serum-free medium. Values are expressed as the mean ± S.D. (n = 4). C, cell adhesion to Ln2-P3 in normal human epidermal keratinocytes (passage 2), normal human dermal fibroblasts (passage 4), CV-1, and NIH/3T3. Ln2-P3 (100 μg/ml) was coated onto plates by drying them for 12 h at room temperature, and cells were allowed to adhere for 30 min in serum-free medium. Values are expressed as the mean ± S.D. (n = 4). D, inhibition of cell adhesion to rLG1 by Ln2-P3. PC12 cells were pretreated with the various peptides (100 μg/ml) or without the peptide (None) for 10 min at room temperature and then seeded on plates precoated with rLG1 (25 μg/ml) for 1 h in serum-free medium. The number of adherent cells was quantified by cell counting. Values are expressed as a percentage of the value for cells pretreated without the peptide (mean ± S.D., n = 4). *, p < 0.01. E, inhibition of cell adhesion to laminin by rLG1. PC12 cells were pretreated with rLG1 (250 and 500 μg/ml) and mutant rLG1 proteins (250 μg/ml) or without the proteins (Vehicle) for 30 min at 37 °C and then seeded on plates precoated with laminin (5 μg/ml) for 30 min in serum-free medium. Cell counting and expression of values were the same as in D. *, p < 0.01. F, inhibition of cell adhesion to laminin by Ln2-P3. PC12 cells were pretreated with the peptides (250 and 500 μg/ml) or without the peptides (Vehicle) for 15 min at 37 °C and then seeded on plates precoated with laminin (5 μg/ml) for 30 min in serum-free medium. Cell counting and expression of values were the same as in D. *, p < 0.01. G, site-directed mutant domains of acidic and/or basic residues in the Ln2-P3 site of laminin α2 LG1 domain. The acidic and basic residues Asp and Arg in the Ln2-P3 site of laminin α2 LG1 domain, indicated by boldface type, were substituted to Ala. H, schematic diagram and SDS-PAGE analysis of the mutant rLG1 proteins in the human laminin α2 chain. The three mutant rLG1 proteins were expressed as His6-tagged fusion proteins. I, adhesion of PC12 cells seeded on plates coated with laminin (LN; 5 μg/ml), rLG1 (25 μg/ml), and three mutant rLG1 proteins (25 μg/ml) for 30 min in serum-free medium. The number of adherent cells was quantified by cell counting. Values are expressed as a percentage from the value of rLG1-treated cells (mean ± S.D., n = 4). *, p < 0.01.