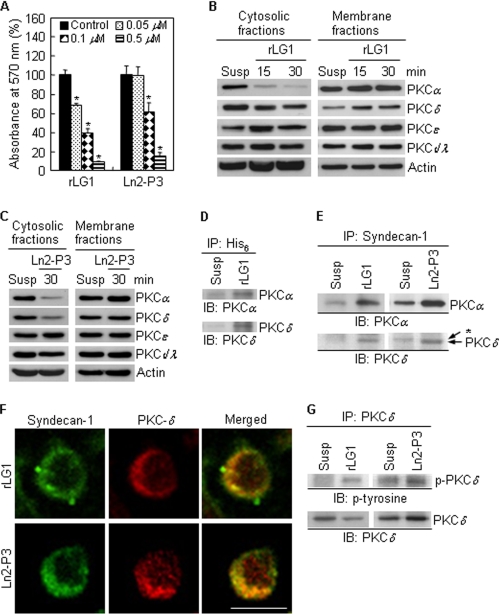
FIGURE 6.
Localization and tyrosine phosphorylation of PKCδ are regulated by LG1 and Ln2-P3. A, inhibition of cell adhesion to rLG1 and Ln2-P3 by calphostin C treatment. PC12 cells were pretreated with calphostin C, an inhibitor for PKC, for 15 min at 37 °C and then seeded on plates precoated with rLG1 and Ln2-P3 for 1 h in serum-free medium. Values are expressed as a percentage of the value for cells pretreated without calphostin C (mean ± S.D., n = 3). *, p < 0.01. B and C, immunoblots of PKC isoforms from cytosolic and membrane fractions of cells cultured on rLG1- or Ln2-P3-coated dishes. PC12 cells were suspended for 15 min and then seeded on rLG1- or Ln2-P3-coated dishes for the indicated times. Susp, suspension of PC12 cells for 45 min. D and E, interactions of His6 and syndecan-1 with PKCα and -δ, respectively, in PC12 cells seeded on rLG1- or Ln2-P3-coated dishes for 30 min. Lysates were subjected to immunoprecipitation and analyzed for PKCα and -δ expression. *, nonspecific signal. IP, immunoprecipitation; IB, immunoblotting. F, colocalization of PKCδ with syndecan-1. PC12 cells were seeded on glass slide chambers precoated with rLG1 or Ln2-P3 for 30 min and immunostained with anti-syndecan-1 antibody (green) and anti-PKCδ antibody (red). Scale bar, 10 μm. G, interaction of PKCδ with phosphotyrosine in PC12 cells seeded on rLG1- or Ln2-P3-coated dishes for 30 min.