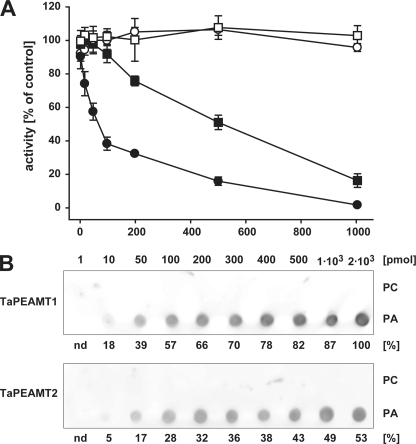
FIGURE 4.
PA inhibition of TaPEAMT activity and PA binding to TaPEAMT isoforms. A, in vitro activity of recombinant TaPEAMT1 (circles) and TaPEAMT2 (squares) in the presence of increasing PA (solid symbols) or PC (open symbols) concentrations, expressed as percentage of activity in control reactions in the absence of lipids. Reactions contained 1 μg of recombinant protein and saturating substrate concentrations (2 mm SAM and 4 mm P-EA; mean ± S.E., n ≥ 2). Although the presence of PC vesicles does not alter the activity of either enzyme, addition of PA vesicles lead to a rapid decrease in TaPEAMT1 activity with an IC50 of ∼70 μm PA, while TaPEAMT2 is only inhibited by very high PA concentrations with an IC50 of ∼470 μm PA. B, specificity of PA binding. 80 nm recombinant TaPEAMT protein was incubated with membranes spotted with concentration series for PC and PA, followed by decoration with primary and secondary antibodies and chemiluminescence detection. Bound TaPEAMT protein can be detected with as little as 10 pmol of PA spotted, whereas PC does not attract any protein to the membrane, even with as much as 2 nmol being spotted. A representative blot selected out of three membranes for each PEAMT isoform in two independent experiments is shown. Numbers below chemiluminescence signals represent the average spot intensity across replicated membranes expressed as the percentage of signal intensity obtained for TaPEAMT1 at the maximal PA concentration spotted.